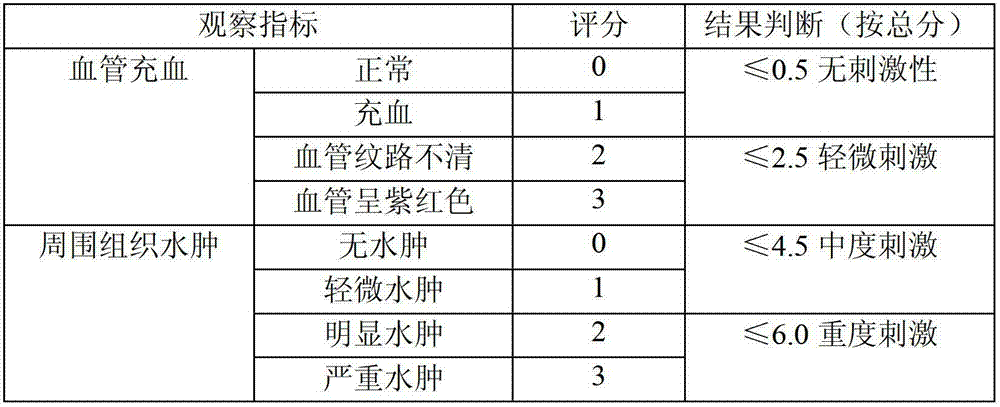
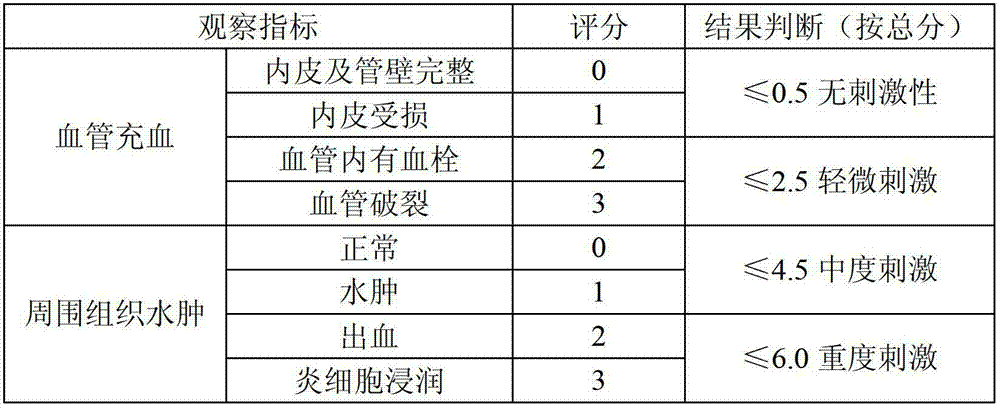
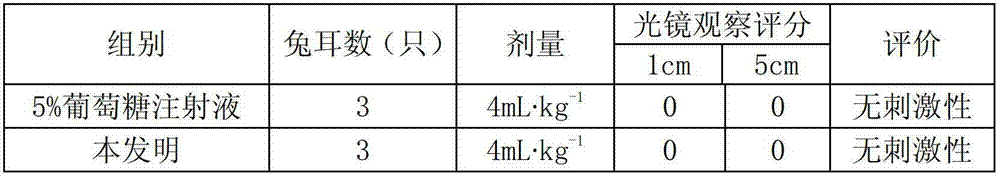
Stable moxifloxacin hydrochloride glucose injection
A technology of moxifloxacin hydrochloride and glucose injection, which is applied in the directions of drug delivery, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of blood calcium drop, precipitation, non-compliance with injection requirements, etc. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Weigh glucose and sodium citrate and dissolve them in water for injection. After stirring to dissolve, dilute hydrochloric acid to adjust the pH value of the solution to 4.3, add moxifloxacin hydrochloride, stir until completely dissolved, then add medicinal charcoal and stir for 10∽30 minutes. Add water for injection and dilute to total amount 1000ml, stir; The weight of moxifloxacin hydrochloride used is to make the concentration of moxifloxacin in the injection be 1.6mg / ml; The weight of glucose is to make the concentration in the injection to be 55mg / ml; the dosage of sodium citrate is 0.1mg / ml; the concentration of adding medicinal charcoal is 0.01mg / ml;
[0054] (2) Coarsely filter the liquid with a titanium filter rod, and then filter the liquid medicine with a 0.22 μm microporous membrane. The filtrate can be checked for foreign matter, incompatible particles, and pH value, etc., and then filled and sealed in a 250ml glass bottle;
[0055] (3) Sterilize afte...
Embodiment 2
[0057] (1) Weigh glucose and sodium citrate and dissolve them in water for injection. After stirring to dissolve, dilute hydrochloric acid to adjust the pH value of the solution to 4.5, add moxifloxacin hydrochloride, stir until completely dissolved, then add medicinal charcoal and stir for 10∽30 minutes. Add water for injection and dilute to a total amount of 1000ml, stir evenly; the weight of moxifloxacin hydrochloride used is to make its concentration in the injection solution be: 5mg / ml; the weight of glucose is to make its concentration in the injection solution be 40mg / ml ml; the dosage of sodium citrate is 0.05mg / ml, and the concentration of adding medicinal charcoal is 0.01mg / ml.
[0058] (2) Coarsely filter the liquid with a titanium filter rod, and then filter the liquid medicine with a 0.22 μm microporous membrane. The filtrate can be checked for foreign matter, incompatible particles, and pH value, etc., and then filled and sealed in a 250ml glass bottle;
[0059] ...
Embodiment 3
[0061] (1) Weigh glucose and sodium citrate and dissolve them in water for injection. After stirring to dissolve, dilute hydrochloric acid to adjust the pH value of the solution to 3.5, add moxifloxacin hydrochloride, stir until completely dissolved, then add medicinal charcoal and stir for 10∽30 minutes. Add water for injection and dilute to a total amount of 1000ml, stir well; the weight of moxifloxacin hydrochloride used is to make its concentration in the injection solution be: 1mg / ml; the weight of glucose is to make its concentration in the injection solution be 50mg / ml ml; the dosage of sodium citrate is 0.03mg / ml, and the concentration of medicinal charcoal is 0.01mg / ml;
[0062] (2) Coarsely filter the liquid with a titanium filter rod, and then filter the liquid medicine with a 0.22 μm microporous membrane. The filtrate can be checked for foreign matter, incompatible particles, and pH value, etc., and then filled and sealed in a 250ml glass bottle;
[0063] (3) Steri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com