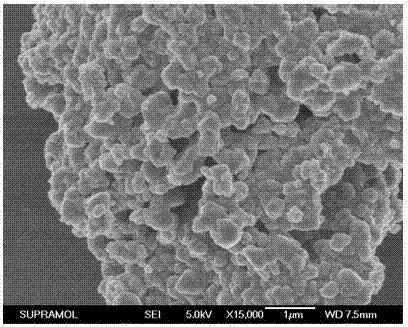
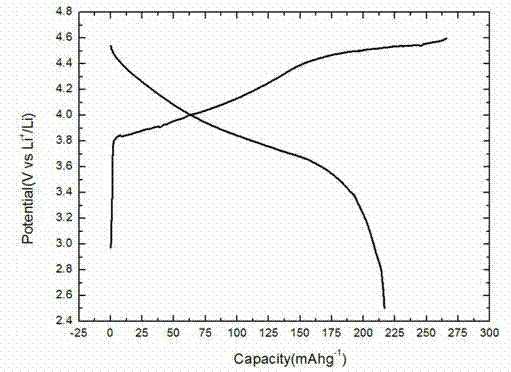
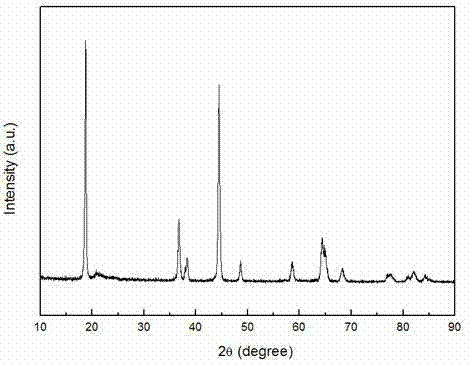
Oxalate coprecipitation preparation method for high-capacity lithium-rich cathode material
A lithium-rich positive electrode material and oxalate technology, which is applied in battery electrodes, nickel oxide/nickel hydroxide, electrical components, etc., can solve the problems such as difficult to accurately control the impurity content and proportion of sediments, and improve structural stability and charge-discharge specific capacity, shape and size are easy, and the effect of low temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Li[Li 0.13 co 0.61 mn 0.26 ]O 2 material, namely x Li 2 MnO 3 ? ( 1-x )LiMO 2 M=Co in the material, x = 0.3.
[0022] (1) Preparation of metal salt solution: Co(NO 3 ) 2 and Mn(NO 3 ) 2 According to the molar ratio n (Co(NO3)2) :n (Mn(NO3)2) =0.61:0.26 ratio dissolved in deionized water, prepared to a concentration of 1mol L -1 solution of metal salts.
[0023] (2) Preparation of oxalate solution: Na 2 C 2 o 4 Dissolved in deionized water, prepared to a concentration of 2.5mol L -1 sodium oxalate solution.
[0024] (3) The solution is mixed and added: adopt the method of "reverse addition", add 1mol L -1 Slowly drop the metal salt solution with a concentration of 2.5mol L -1 In the sodium oxalate solution, the pH value of the mediation solution is 6.5, forming a co-precipitation solution.
[0025] (4) Solid-liquid separation: filter the above co-precipitation solution through filter paper, wash with dei...
Embodiment 2
[0028] Preparation of Li[Li 0.184 Ni 0.224 mn 0.725 ]O 2 material, namely x Li 2 MnO 3 ? ( 1-x )LiMO 2 M= Ni in the material 1 / 2 mn 1 / 2 , x = 0.45 .
[0029] (1) Preparation of metal salt solution: NiSO 4 and MnSO 4 According to the molar ratio n (NiSO4) :n (MnSO4) =0.224:0.725 ratio dissolved in deionized water, prepared to a concentration of 2.5mol L -1 solution of metal salts.
[0030] (2) Preparation of oxalate solution: K 2 C 2 o 4 Dissolved in deionized water, prepared to a concentration of 3.5mol L -1 of potassium oxalate solution.
[0031] (3) The solution is mixed and added: adopt the method of "adding together", add 2.5mol·L -1 The metal salt solution and the concentration is 3.5mol L -1 The potassium oxalate solution was dropped into a beaker equipped with deionized water at the same time, and the pH value of the mediation solution was 8 to form a co-precipitation solution.
[0032] (4) Solid-liquid separati...
Embodiment 3
[0036] Preparation of Li[Li 0.133 Ni 0.3 mn 0.567 ]O 2 material, namely x Li 2 MnO 3 ? ( 1-x )LiMO 2 M= Ni in the material 1 / 2 mn 1 / 2 , x = 0.3 .
[0037] (1) Preparation of metal salt solution: NiSO 4 and Mn(NO 3 ) 2 According to the molar ratio n (NiSO4) :n (Mn(NO3)2) The ratio of =0.300:0.567 is dissolved in deionized water, and the concentration is 3mol L -1 solution of metal salts.
[0038] (2) Preparation of oxalate solution: add NH 4 HC 2 o 4 Dissolved in deionized water, prepared to a concentration of 3mol L -1 ammonium hydrogen oxalate solution.
[0039] (3) The solution is mixed and added: adopt the method of "reverse addition", add 3mol L -1 Slowly drop the metal salt solution with a concentration of 3mol L -1 In the ammonium hydrogen oxalate solution, the pH value of the mediation solution is 7, forming a co-precipitation solution.
[0040] (4) Solid-liquid separation: filter the above co-precipitation sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com