Boric acid compound, positive electrode for secondary battery, and method for manufacturing secondary battery
A technology of boric acid compound and manufacturing method, which is applied in electrode manufacturing, boron compound, boron oxide compound, etc., can solve the problems of increased manufacturing cost and large amount of gas generated, and achieves the effect of excellent reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11)
[0154] Weigh ferric oxide (Fe 3 o 4 ), manganese dioxide (MnO 2 ), cobalt tetroxide (Co 3 o 4 ), nickel oxide (NiO) and boron oxide (B 2 o 3 ), making it the composition of the first compound shown in Table 1, respectively, to obtain a raw material mixture, which was pulverized by dry method. These pulverized materials were respectively filled in platinum alloy crucibles containing 20% by mass rhodium. Next, this crucible was placed in an electric furnace (manufactured by Motoyama Co., Ltd., device name: NH3045F) equipped with a heating element made of molybdenum silicide. Make N at a flow rate of 2L / min 2 The electric furnace was heated at 1350° C. for 0.5 hour while the gas was circulated. It was confirmed visually that it became transparent, and each melt was obtained.
[0155] Next, the melt in the crucible was passed through double rollers made of stainless steel with a diameter of about 15 cm at 400 revolutions per minute, thereby 5 °C / sec speed cooling to ob...
Embodiment 12~13)
[0160] with Fe 2 B 2 o 5 is the first compound, Li 2 CO 3 As the second compound, it was pulverized in the same manner as in Example 1 so that the molar ratio based on these oxides was 1:0.8 (Example 12) and 1:1.2 (Example 13), and the pulverized product was divided into 3% by volume h 2 -Ar gas, heated at 600°C for 8 hours to obtain 0.8 MB O 2.9 and Li 1.2 MB O 3.1 Boronic acid compound particles of the composition shown.
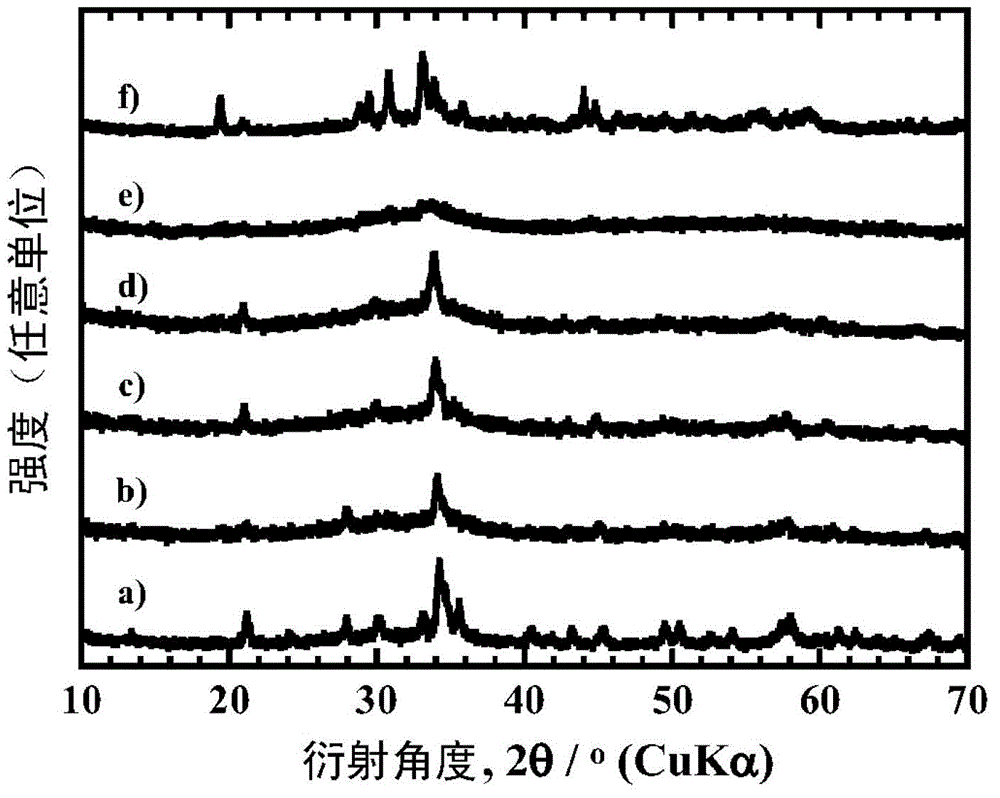
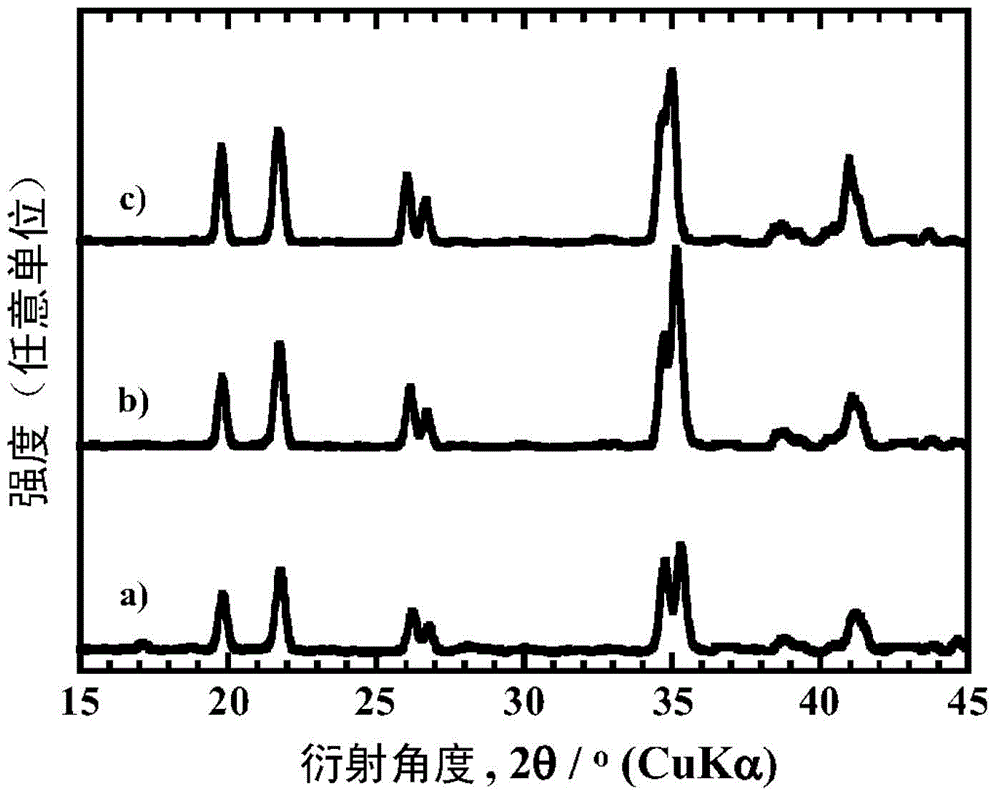
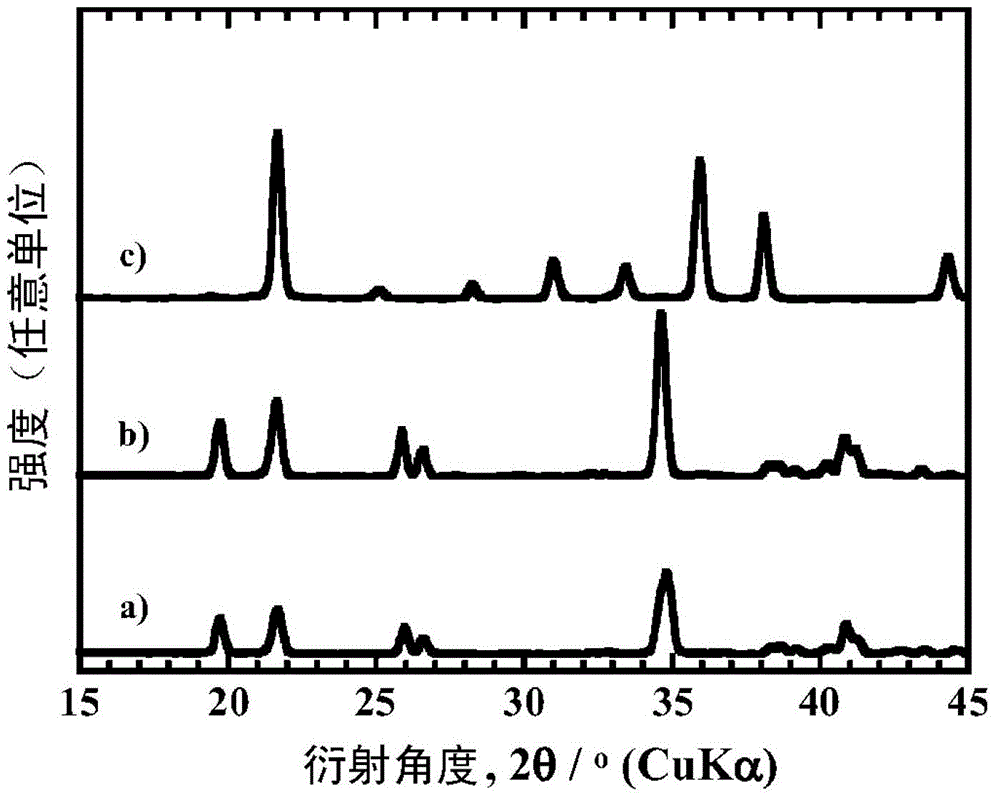
[0161] When identifying the mineral phase of each particle obtained with an X-ray diffraction device, the particles obtained in Examples 1 to 8, 12 and 13 obtained a similarity to that of the existing LiFeBO 3 (PDF number 01-070-8321) and / or LiMnBO 3 (PDF number 01-053-0371) similar to the diffraction pattern. In addition, the particles obtained in Examples 9-11 obtained the same properties as the existing LiFeBO 3 (PDF number 01-070-8321) similar to the diffraction pattern. LiMBO obtained by heating at 600°C for 8 hours in Examples 1 to 6 3 ...
Embodiment 14~19)
[0165] The flaky cured products obtained in Examples 1 to 6 were preliminarily crushed by dry method, and were blended with lithium carbonate (the second compound) so that the molar ratio based on the oxide was 1:1, thereby obtaining a blend, and then, Carbon black was added to the blend so that the mass ratio of the blend to carbon black was 90:10. This was pulverized by a wet method in the same manner as in Example 1. Put each pulverized product in N 2 In gas, heating at 600°C for 8 hours, thus obtaining carbon-containing, LiMBO 3 Boronic acid compound pellets of the indicated composition.
[0166] When using the X-ray diffraction device to identify the mineral phases of the obtained particles, all obtained the same as the existing LiFeBO 3 (PDF number 01-070-8321) and / or LiMnBO 3 (PDF number 01-053-0371) similar to the diffraction pattern. Furthermore, when measuring the carbon content of the boric acid compound particles obtained in Examples 14, 16 and 19 with a carbo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Median particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com