Composition for formation of resist underlayer film containing silicon having nitrogen-containing ring
A resist lower layer and resist film technology, which is applied in the fields of compounds, coatings, organic chemistry of elements of group 4/14 of the periodic table, and can solve problems such as the problem of large active rays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
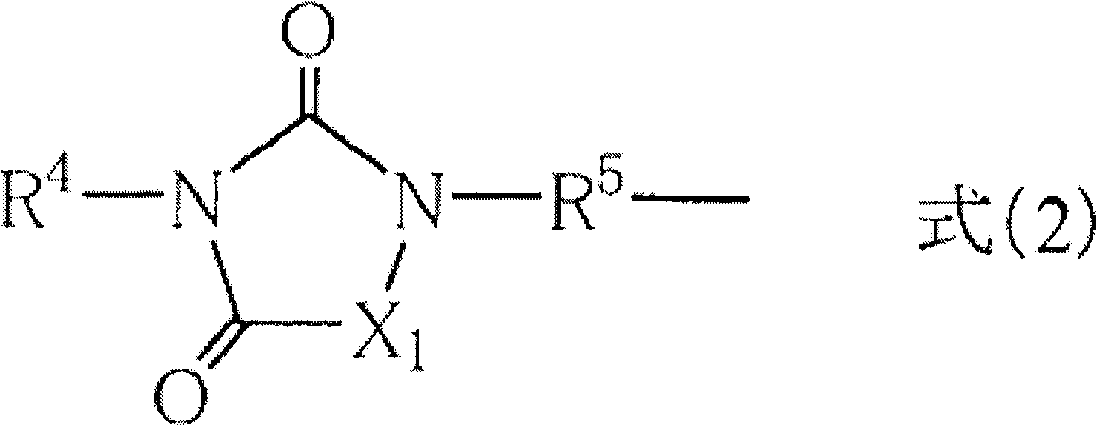
[0252] 30.0 g (0.1774 moles) of monoallyl isocyanurate, 36.42 g (0.2217 moles, 1.25 equivalents to 1 mole of vinyl), chloroplatinic acid (VI ) hydrate 0.09g, toluene 100ml, and reacted at 100°C for 6 hours. Then, toluene, excess contained triethoxysilane were removed by an evaporator. Then, extraction was performed with 100 ml of dichloromethane and 50 ml of distilled water x 3, dehydrated with magnesium sulfate, and the dichloromethane was removed by an evaporator to obtain a crude product. The obtained crude product was purified by distillation to obtain a compound represented by formula (E-2) as a target substance.
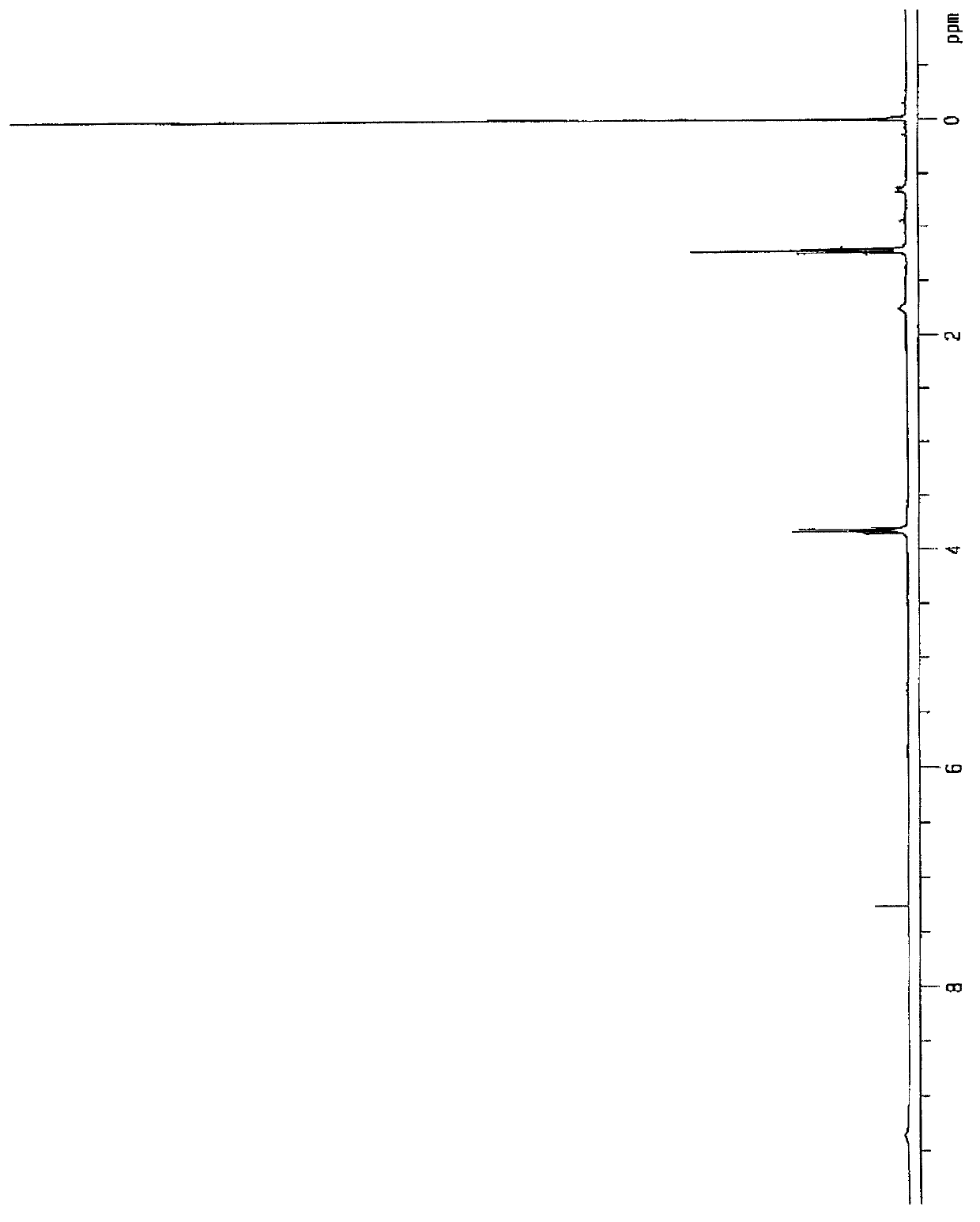
[0253] Regarding the resulting compound, by 1 H-NMR measurement was identified. The measurement was performed under the following conditions: sample tube: 5 mm, solvent: deuterated chloroform, measurement temperature: room temperature, pulse interval: 5 seconds, number of accumulations: 32 times, reference sample: tetramethylsilane (TMS).
[0254] 1 H-NMR ...
Synthetic example 2
[0257] 10.00 g of monoallyl diglycidyl isocyanurate and 60.00 g of toluene were added to a 200 ml four-necked flask equipped with an electromagnetic stirrer, and stirred at room temperature. Next, after adding 700 μl of Karstedt catalyst (platinum (0)-1,1,3,3-tetramethyldisiloxane complex 0.1 M xylene solution), 7.1 ml of triethoxysilane was added dropwise, and the Stir at room temperature for 19 hours. After the reaction, the reaction solution was concentrated and dried to obtain a crude product. Distillation under reduced pressure was carried out at an external temperature of 240° C. / pressure of 0.7 torr to obtain 13.46 g (85%) of the compound represented by the formula (E-3).
[0258] The measurement conditions of NMR were performed in the same manner as above.
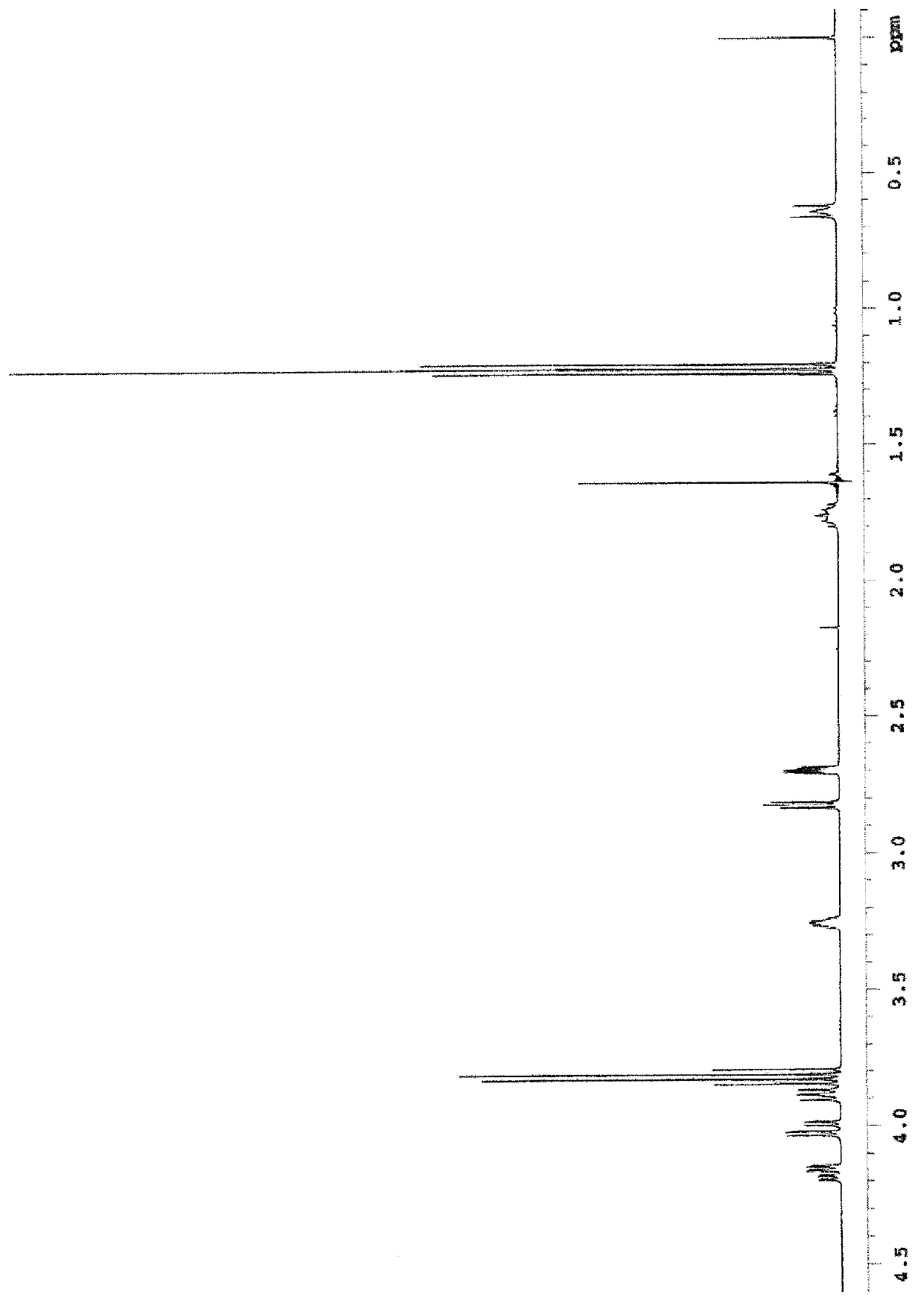
[0259] 1 H-NMR(400MHz)inCDCl 3 : 0.62~0.67ppm (m, 2H), 1.22ppm (t, J=7.0Hz, 9H), 1.73~1.79ppm (m, 2H), 2.68~2.71ppm (m, 2H), 2.82ppm (dd, J =4.9Hz, 4.0Hz, 2H), 3.23~3.28ppm(m, 2H), 3.81ppm(q, J=7.0Hz, 6H), 3.8...
Synthetic example 3
[0262] Add 10.00 g of dimethyl monoallyl isocyanurate, 24.18 g of (3-mercaptopropyl) triethoxysilane, 0.21 g of azobisisobutyronitrile, and Base ketone 100ml, carry out reaction at 95 ℃ for 6 hours. Then, methyl ethyl ketone was removed by an evaporator. Then, extraction was performed with 100 ml of dichloromethane and 50 ml of distilled water x 3, dehydrated with magnesium sulfate, and the dichloromethane was removed by an evaporator to obtain a crude product. The obtained crude product was purified by distillation to obtain a compound represented by formula (E-4) as a target substance.
[0263] The measurement conditions of NMR were performed in the same manner as above.
[0264] 1 H-NMR (400MHz): 0.71~0.75ppm(t, 2H), 1.20~1.25ppm(t, 9H), 1.65~1.73ppm(quint, 2H), 1.90~1.98ppm(quint, 2H), 2.53~2.57 ppm(m, 4H), 3.35ppm(s, 6H), 3.79~3.85ppm(quartet, 6H), 3.98~4.02ppm(t, 2H)
[0265]
PUM
| Property | Measurement | Unit |
|---|---|---|
| aperture size | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com