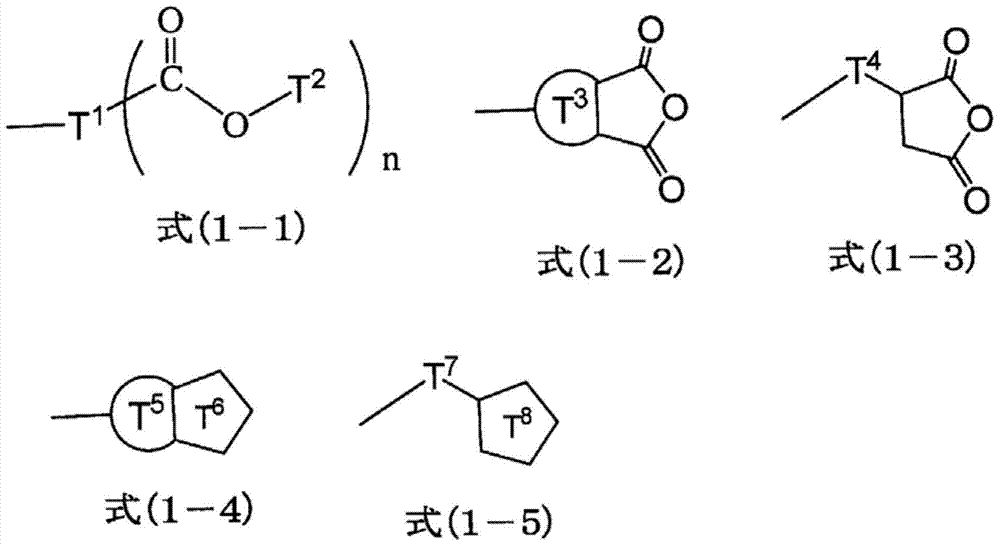
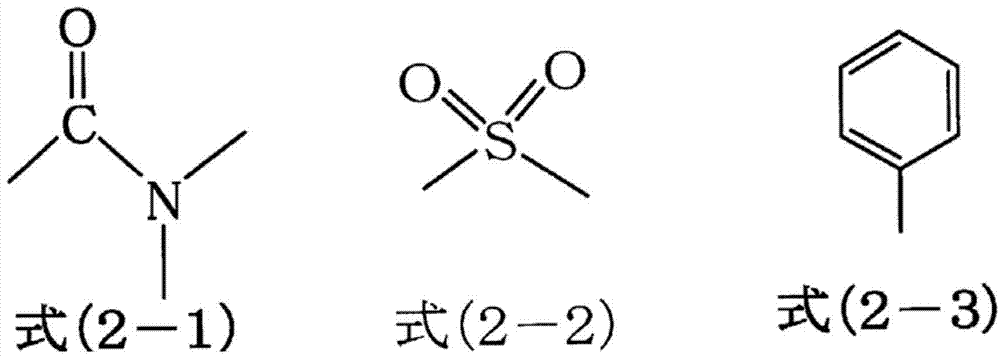
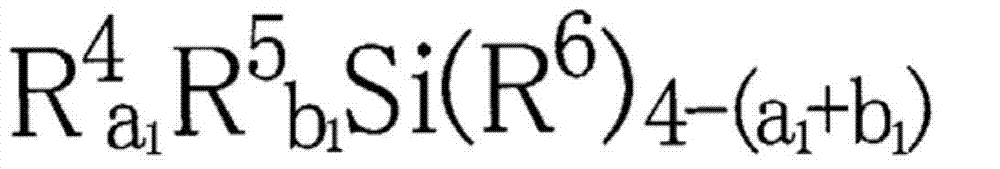Ester-group-containing composition for forming silicon-containing resist underlayer film
一种抗蚀剂下层、组合物的技术,应用在形成下层膜的组合物领域,能够解决反射的影响大问题等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0217] (Synthesis of compound 1)
[0218]
[0219]Add 15.00 g of 5-norbornene-2-methyl carboxylate, 3.76 g of Karstedt's catalyst (platinum (0)-1,3-divinyl- 2% by mass xylene solution of 1,1,3,3-tetramethyldisiloxane complex), 112 g of toluene, and 17.81 g of triethoxysilane were added dropwise over 10 minutes. After stirring at room temperature for 5 hours, the reaction liquid was concentrated to dryness, and the obtained crude product was purified by vacuum distillation to obtain Compound 1.
[0220] DMSO-d 6 middle 1 H-NMR (500MHz): 0.62~0.79ppm(m, 1H), 1.12~1.79ppm(m, 15H), 2.20~2.50ppm(m, 3H), 3.57ppm(q, 3H), 3.70~3.77ppm( m, 6H)
[0221] (Synthesis of Compound 2)
[0222]
[0223] Add 15.00 g of 3,3-dimethyl-4-pentenoic acid methyl ester, 1.91 g of Karstedt's catalyst (platinum (0)-1,3- 2% by mass xylene solution of divinyl-1,1,3,3-tetramethyldisiloxane complex), 112 g of toluene, and 19.06 g of triethoxysilane were added dropwise over 10 minutes. After stir...
Synthetic example 1
[0242] 15.40g (75mol% in all silanes) of tetraethoxysilane, 1.23g (7mol% in all silanes) of methyltriethoxysilane, 1.37g (7mol% in all silanes) phenyltrimethoxysilane, 1.72g (6mol% in all silanes) of methylsulfonylmethylphenyltrimethoxysilane, 1.62g (5mol% in all silanes) of (5-(tri Ethoxysilyl) norbornene-2,3-dicarboxylic anhydride, 32.00g of acetone were added to a 100ml flask, while the mixed solution was stirred with a magnetic stirrer, 6.66g of 0.01mol / l hydrochloric acid was added Added dropwise in the mixed solution. After the addition, the flask was moved to an oil bath adjusted to 85°C, and reacted for 240 minutes under heating and reflux. Then, the reaction solution was cooled to room temperature, and propylene glycol monomethyl ether ethyl was added in the reaction solution Ethyl acid ester 21g, ethanol, water, hydrochloric acid decompression distillation and concentration as reaction by-product, obtain the propylene glycol monomethyl ether acetate solution of hydro...
Synthetic example 2
[0244] 15.48g (75mol% in all silanes) of tetraethoxysilane, 1.24g (7mol% in total silanes) of methyltriethoxysilane, 1.37g (7mol% in total silanes) phenyltrimethoxysilane, 1.73g (6mol% in all silanes) of methylsulfonylmethylphenyltrimethoxysilane, 1.51g (5mol% in total silanes) of 3-(triethyl Oxysilyl) propyl succinic anhydride and 31.98g of acetone were added to a 100ml flask, and while the mixed solution was stirred with a magnetic stirrer, 6.69g of 0.01mol / l hydrochloric acid was added dropwise to the mixed solution. After the addition, the flask was moved to an oil bath adjusted to 85° C., and the mixture was reacted under reflux under heating for 240 minutes. Then, the reaction solution was cooled to room temperature, 21 g of propylene glycol monomethyl ether acetate was added to the reaction solution, and ethanol, water, and hydrochloric acid as reaction by-products were distilled off under reduced pressure and concentrated to obtain a hydrolysis condensate (polymer). P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com