Synthetic method of repaglinide
A synthesis method and methyl technology, which are applied in the field of synthesis technology of repaglinide, can solve the problems of large reaction temperature change, great harm to human body, equipment corrosion, etc., and achieve short reaction time, high product yield, and improved yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
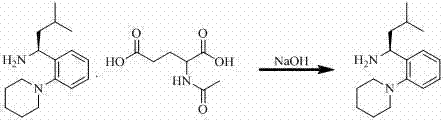
[0027] Embodiment 1: a, ( S Preparation of )-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine
[0028] Add 0.01mol (4.35g) of ( S )-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine N - Dissolve acetylglutamate in 50ml of dichloromethane, stir and cool down to 15°C, then add 2.5ml of 4M NaOH solution dropwise, when the material is clear, extract three times with 25ml of dichloromethane, combine the extracts, add Anhydrous NaSO 4 dry to get ( S )-3-Methyl-1-[2-(1-piperidinyl)phenyl]butylamine in dichloromethane;
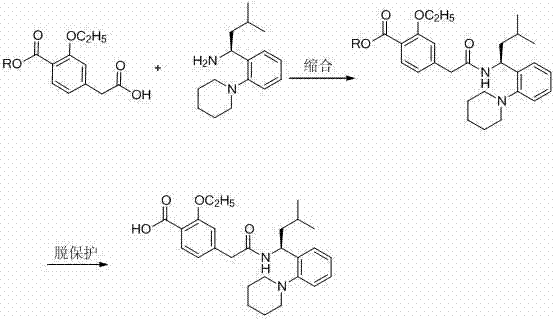
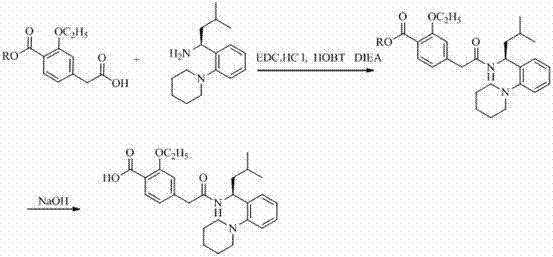
[0029] b, 2-ethoxy-4-[2-[[( S )-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]ethyl benzoate
[0030] The above 75ml ( S )-3-Methyl-1-[2-(1-piperidinyl)phenyl]butylamine in dichloromethane was stirred and cooled to 15°C, and then 0.015mol (3.78g) of 3-ethoxy- 4-ethoxycarbonylphenylacetic acid, 0.015mol (2.03g) of HOBT, 0.015mol (5.55g) of DIEA and 0.015mol (1.94g) of EDC·HCl, and monitor the reaction process by thin-layer chromatography (TLC), 4-6 hou...
Embodiment 2
[0033] Embodiment 2: the difference between this embodiment and embodiment 1 is that
[0034] a.( S Preparation of )-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine
[0035] 0.015mol (6.53g) of ( S )-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine N - Dissolve acetylglutamate in 70ml of dichloromethane, stir and cool down to 20°C, then add 7.5ml of 2M KOH aqueous solution dropwise, when the feed liquid is clear, extract three times with 30ml of dichloromethane, combine the extracts, add Anhydrous NaSO 4 dry to get ( S )-3-Methyl-1-[2-(1-piperidinyl)phenyl]butylamine in dichloromethane;
[0036] b, 2-ethoxy-4-[2-[[( S )-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]ethyl benzoate
[0037] The above 80ml ( S )-3-Methyl-1-[2-(1-piperidinyl)phenyl]butylamine in dichloromethane was stirred and cooled to 20°C, then 0.02mol (5.05g) of 3-ethoxy- 4-ethoxycarbonylphenylacetic acid, 0.02mol (2.70g) of HOBT, 0.02mol (2.58g) of DIEA and 0.02mol (3.83g) of EDC·HCl, TLC ...
Embodiment 3
[0040] Embodiment 3: the difference between this embodiment and embodiment 1 is,
[0041] a.( S Preparation of )-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine
[0042] 0.015mol (6.53g) of ( S )-3-methyl-1-[2-(1-piperidinyl)phenyl]butylamine N - Dissolve acetylglutamate in 60ml of dichloromethane, stir and cool down to 0°C, then add 4M NH 3 ·H 2 O solution 3.75ml, when the material liquid is clarified, extract three times with 30ml dichloromethane, after combining the extracts, add anhydrous NaSO 4 dry to get ( S )-3-Methyl-1-[2-(1-piperidinyl)phenyl]butylamine in dichloromethane;
[0043] b, 2-ethoxy-4-[2-[[( S )-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]ethyl benzoate
[0044] The above 80ml ( S )-3-Methyl-1-[2-(1-piperidinyl)phenyl]butylamine in dichloromethane was stirred and cooled to 20°C, then 0.02mol (5.05g) of 3-ethoxy- 4-ethoxycarbonylphenylacetic acid, 0.02mol (2.70g) of HOBT, 0.02mol (2.58g) of DIEA and 0.02mol (3.83g) of EDC·HCl, TLC mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com