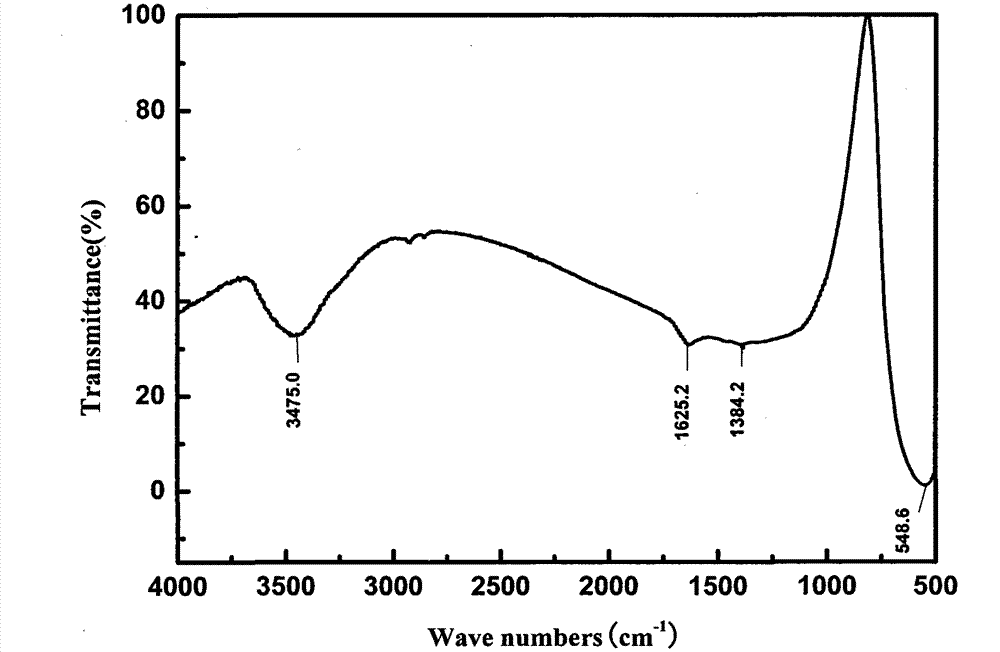
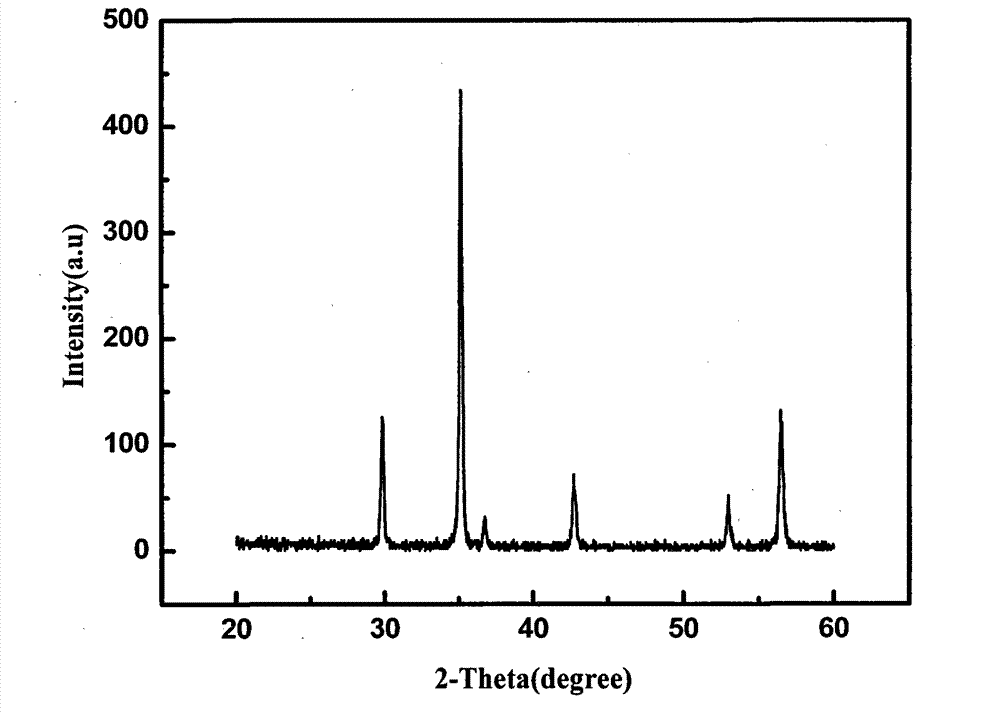
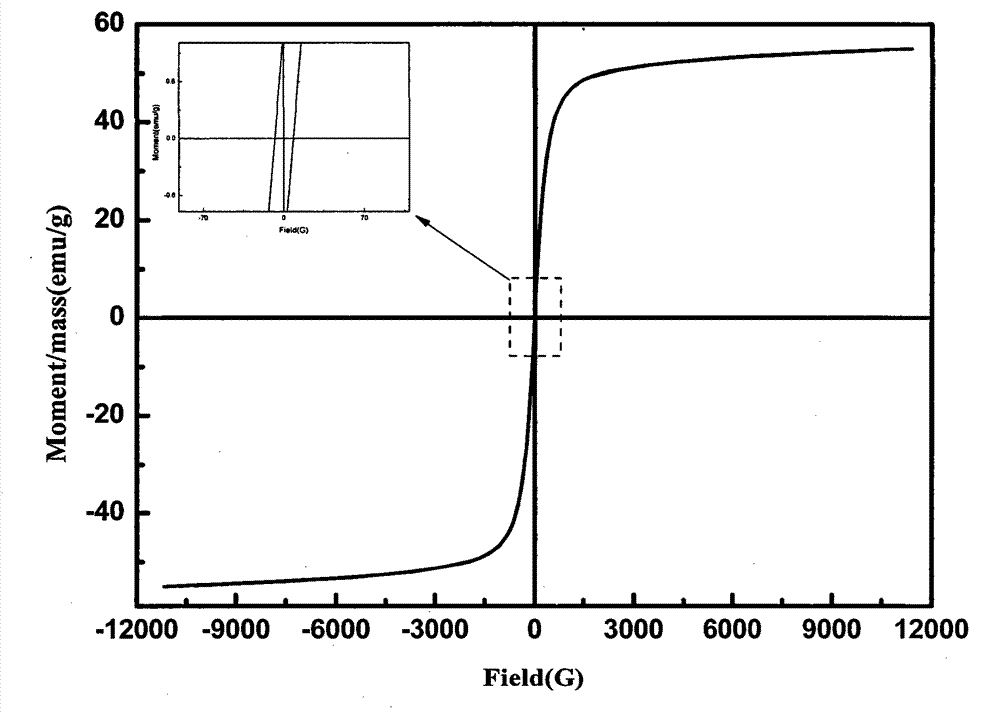
Method for preparing manganese-zinc ferrites through carrying out acid dipping on low-grade manganese ores and carrying out ferromanganese remaining and impurity removal
A low-grade manganese ore and manganese-zinc ferrite technology, applied in the field of manganese-zinc ferrite preparation, can solve the problems of increasing production costs, increasing production costs, unfavorable scale production, etc., and achieve reduced emissions, less equipment, and raw material costs low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for preparing manganese-zinc ferrite by acid leaching of low-grade manganese ore and ferromanganese preservation for impurity removal, the specific steps are as follows:
[0026] (1) extract and prepare manganese sulfate and ferric sulfate solution
[0027] Using low-grade manganese ore as raw material, first crush the low-grade manganese ore through a pulverizer, and then sieve it with a sieve with an aperture of 0.074mm. For the sieved manganese ore powder, the mass (g) of the manganese ore powder: the volume (mL) ratio of the sulfuric acid leaching agent with a mass concentration of 30% is a ratio of 1: 3, the manganese ore powder and the sulfuric acid leaching agent are added In the first reaction vessel, heat it with a 90°C water bath, stir and leaching with a stirrer for 2.5 hours, stop heating and stirring, cool to room temperature, and filter for the first time with a filter to collect the filtrate and filter residue respectively. The filter residue is...
Embodiment 2
[0034] A method for preparing manganese-zinc ferrite by acid leaching of low-grade manganese ore and ferromanganese preservation for impurity removal, the specific steps are as follows:
[0035] In the 1st (1) step: the mass concentration of sulfuric acid leaching agent is 20%, the quality (g) of manganese ore powder: the volume (mL) ratio of sulfuric acid leaching agent is 1: 2, and the water bath temperature is 80 ℃, stirring and leaching Take the time to be 2.0h, adjust the pH value of the filtrate collected by the first filtration with sodium hydroxide solution to be 5.30, the quality (g) of reduced iron powder: the volume (mL) ratio of the filtrate collected by the first filtration is 1: 8. The stirring time is 20 min, the mass (g) of sodium fluoride: the volume (mL) ratio of the filtrate collected by the second filtration is 1:8, and the stirring time is 10 min.
[0036] In step (2): NH 4 HCO 3 The mass (g): the volume (mL) of the mixed solution of manganese sulfate an...
Embodiment 3
[0040] A method for preparing manganese-zinc ferrite by acid leaching of low-grade manganese ore and ferromanganese preservation for impurity removal, the specific steps are as follows:
[0041] In the 1st (1) step: the mass concentration of sulfuric acid leaching agent is 40%, the quality (g) of manganese ore powder: the volume (mL) ratio of sulfuric acid leaching agent is 1: 4, and the water bath temperature is 100 ℃, stirring and leaching Take time as 3.0h, adjust the pH value of the filtrate collected by filtration for the first time with sodium hydroxide solution to be 5.34, the quality (g) of reduced iron powder: the volume (mL) ratio of the filtrate collected by filtration for the first time is 1: 10. The stirring time was 40 min, the mass (g) of sodium fluoride: the volume (mL) ratio of the filtrate collected by the second filtration was 1:10, and the stirring time was 30 min.
[0042] In step (2): NH 4 HCO 3 The mass (g): the volume (mL) of the mixed solution of man...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com