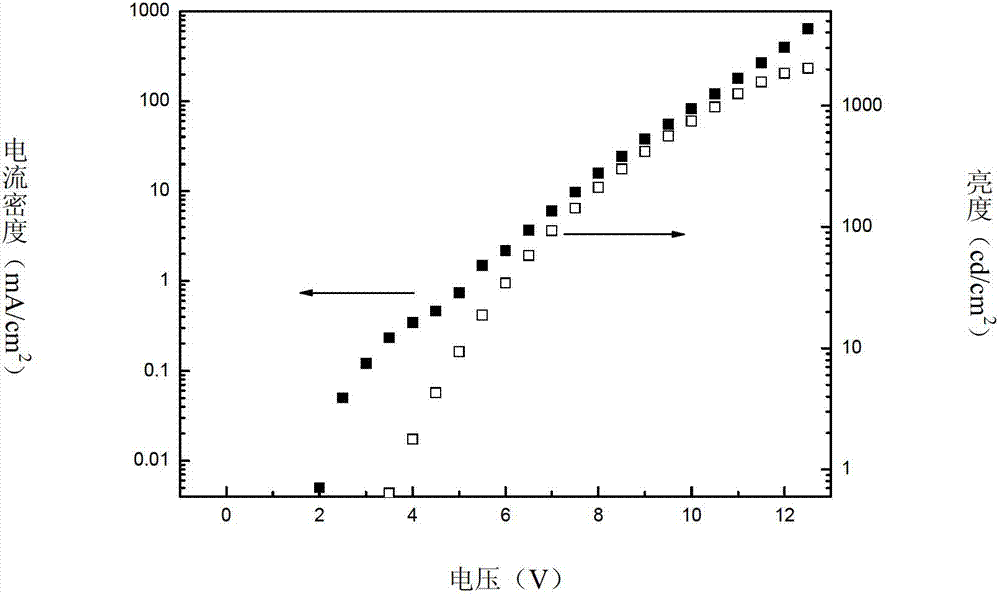
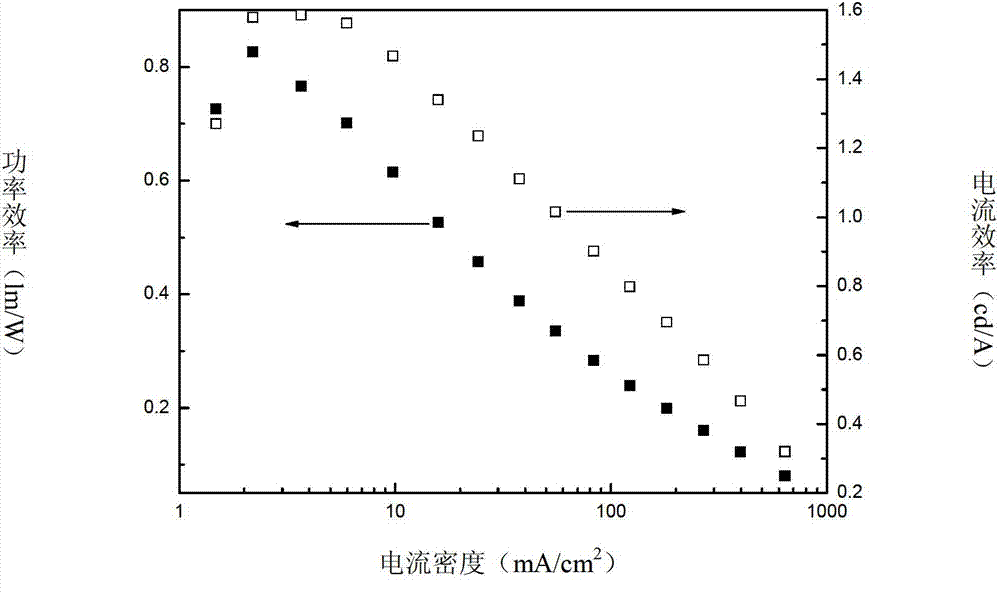
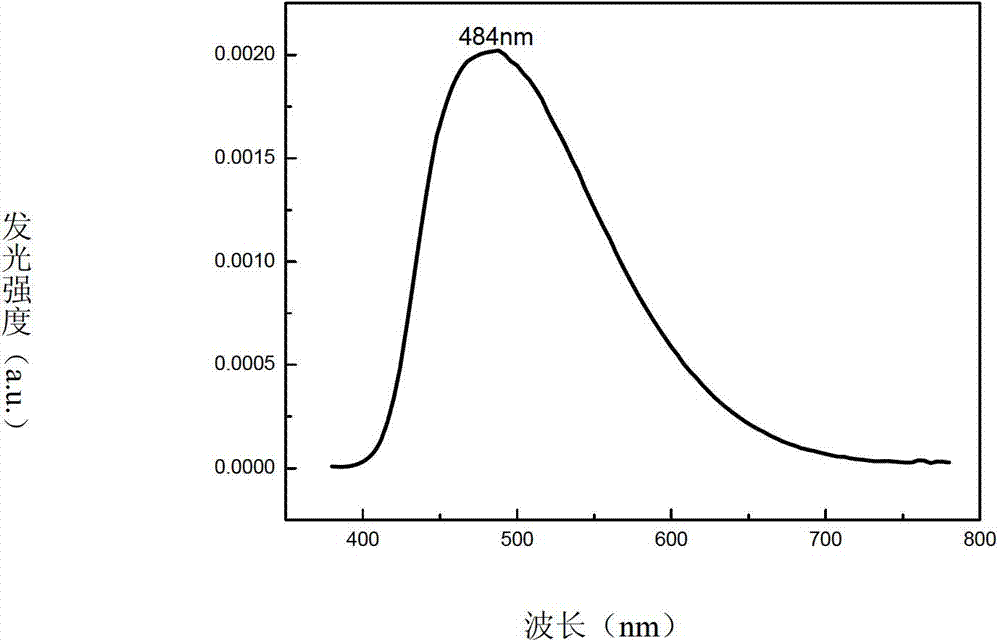
Novel bipolar material and application thereof
A bipolar, new technology, applied in the direction of luminescent materials, electrical components, circuits, etc., can solve the problem of not very high, and achieve the effects of high glass transition temperature and decomposition temperature, high color purity, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 2,7-bis(9-phenyl-9H-carbazol-3-yl)dibenzothiophene sulfone (compound 1)
[0056] Add starting materials 3.74g 2,7-dibromodibenzothiophene sulfone (10mmol) and 3.16g (9-phenyl-9H-carbazol-3-yl)boronic acid (11mmol) into a three-necked flask, and dissolve them in a mixed solvent (100mL toluene, 50mL ethanol), then add 50mL Na 2 CO 3 Aqueous solution (2M), nitrogen gas stirred for 1h to remove the oxygen in the reaction flask. Then add Pd(PPh 3 ) 4 0.24g (0.21mmol), reflux under vigorous stirring, the reaction process is controlled by thin-layer chromatography. After reacting for 24 hours, add 50 mL of deionized water to the reaction solution, filter to remove insoluble matter, separate the water phase from the organic phase, extract the water phase with ethyl acetate (20 mL each time, 3 times), mix the organic phase and reduce pressure Concentrate by distillation to about 10mL, and separate by column chromatography. The eluent is ethyl acetate:n-hexane=1:1...
Embodiment 2
[0058] Example 2 2,7-bis(6-tert-butyl-9-phenyl-9H-carbazol-3yl)dibenzothiophene sulfone (compound 2)
[0059] Using 2,7-dibromodibenzothiophene sulfone and (6-tert-butyl-9-phenyl-9H-carbazol-3-yl)boronic acid as starting materials according to the synthetic method of compound 1 in Example 1 .
[0060] MS (m / z): 811.5. Elemental analysis (C 56 h 46 N 2 o 2 S): theoretical value C: 82.93, H: 5.72, N: 3.45, measured value C: 82.88, H: 5.79, N: 3.52.
Embodiment 3
[0061] Example 3 2,7-bis(6-methoxy-9-phenyl-9H-carbazol-3-yl)dibenzothiophene sulfone (compound 3)
[0062] Using 2,7-dibromodibenzothiophene sulfone and (6-methoxy-9-phenyl-9H-carbazol-3-yl)boronic acid as starting materials according to the synthetic method of compound 1 in Example 1 .
[0063] MS (m / z): 759.6. Elemental analysis (C 50 h 34 N 2 O4S): theoretical value C: 79.13, H: 4.52, N: 3.69, measured value C: 79.19, H: 4.69, N: 3.57.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com