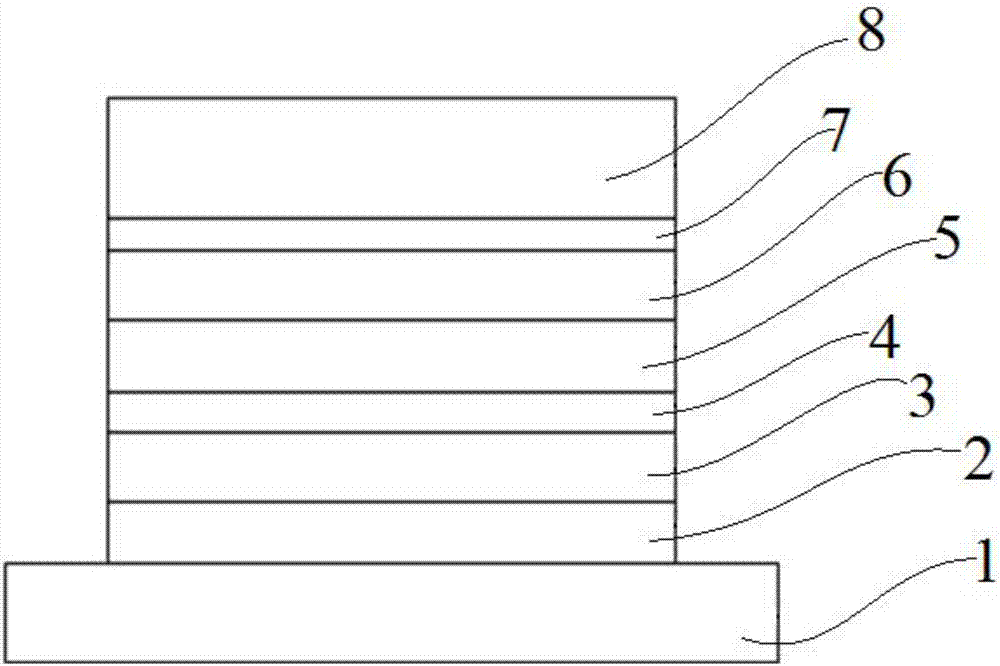
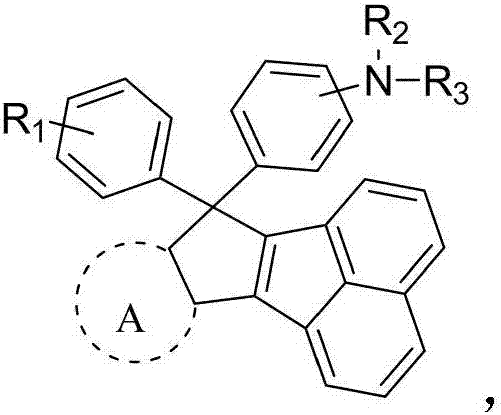
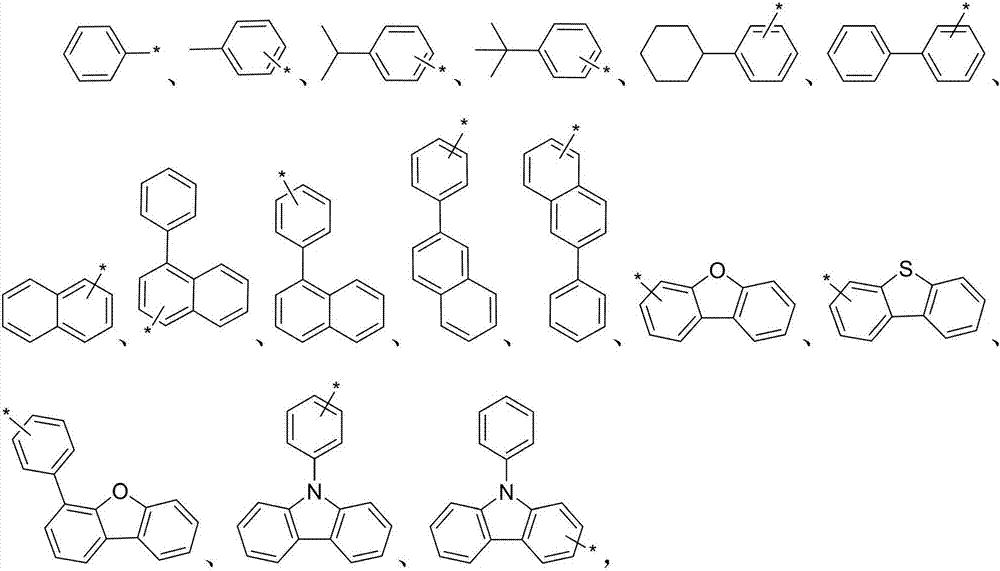
Screw-ring hole-transporting material, and preparation method and application thereof
A technology of hole transport materials and spiro rings, which is applied in the direction of luminescent materials, carboxylic acid nitrile preparation, amino compound preparation, etc. It can solve the problems of blocking the full practical application of OLED technology, limiting the luminous efficiency, service life and operating voltage of devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 compound 1
[0058] (1) Preparation of Compound II:
[0059]
[0060] Get raw material 23.1g 1-bromoacenaphthylene (100.0mmol), 14.7g potassium acetate (150mmol) 30.5 double pinacol borate (120mmol) and join in 250g toluene, under the protection of nitrogen, add catalyst 0.8g Pd (dppf)Cl 2 (1mmol), then be heated to reflux, select water separator to divide water in the reaction process, adopt thin-layer chromatography (TLC) to track the reaction process simultaneously, after about 5.0 hour reaction finishes, add 200g water, system layering, then wash with water Finally, the organic phase was dried over anhydrous sodium sulfate, and then purified by column chromatography with toluene to obtain 23.1 g of compound II with a yield of 83.01%.
[0061] (2) Preparation of Compound IV:
[0062]
[0063] Get the compound compound II (12.5g, 45.0mmol), m-bromoiodobenzene (15.3g, 54.0mmol), 12.4g potassium carbonate (90mmol), 50g water and ...
Embodiment 2
[0073] The preparation of embodiment 2 compound 15
[0074] (1) Preparation of Compound IV:
[0075]
[0076] Take the obtained compound II (12.5g, 45.0mmol), 2-bromo-3-iodonaphthalene (16.6g, 50.0mmol), 12.4g potassium carbonate (90mmol), 50g water and 140g toluene, under the protection of nitrogen, Add catalyst 0.5g Pd(PPh 3 ) 4 (0.45mmol), then heated to reflux, while adopting thin-layer chromatography (TLC) to track the reaction process, after about 12.0 hours of reaction, after being down to room temperature, the system was layered, and after washing with water, the organic phase was decompressed and decompressed. Solvent until there is no distillate, and then recrystallize from toluene ethanol to obtain 13.0 g of compound IV with a yield of 81.25%.
[0077] (2) Preparation of compound VI:
[0078]
[0079] Compound IV (12.5 g, 35.0 mmol) obtained in step (1) was added to 100 g of THF solution, under the protection of nitrogen, the temperature was lowered to -78...
Embodiment 3
[0086] The preparation of embodiment 3 compound 16
[0087] (1) Preparation of Compound IV:
[0088]
[0089] Take the obtained compound II (12.5g, 45.0mmol), 1-bromo-2-iodonaphthalene (16.6g, 50.0mmol), 12.4g potassium carbonate (90mmol), 50g water and 140g toluene, under the protection of nitrogen, Add catalyst 0.5g Pd(PPh 3 ) 4 (0.45mmol), then heated to reflux, while adopting thin-layer chromatography (TLC) to track the reaction process, after about 12.0 hours of reaction, after being down to room temperature, the system was layered, and after washing with water, the organic phase was decompressed and decompressed. Solvent until there is no distillate, and then recrystallize with toluene ethanol to obtain 12.2 g of compound IV with a yield of 76.25%.
[0090] (2) Preparation of compound VI:
[0091]
[0092] Compound IV (12.5 g, 35.0 mmol) obtained in step (1) was added to 100 g of THF solution, under the protection of nitrogen, the temperature was lowered to -78...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com