Bipolar organic electroluminescent material and preparation method thereof
An electroluminescent material, bipolar technology, applied in the direction of luminescent materials, chemical instruments and methods, can solve the problems of difficulty in taking into account hole and electron transport efficiency, poor electroluminescence efficiency, etc., to promote transport and injection, The effect of high color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
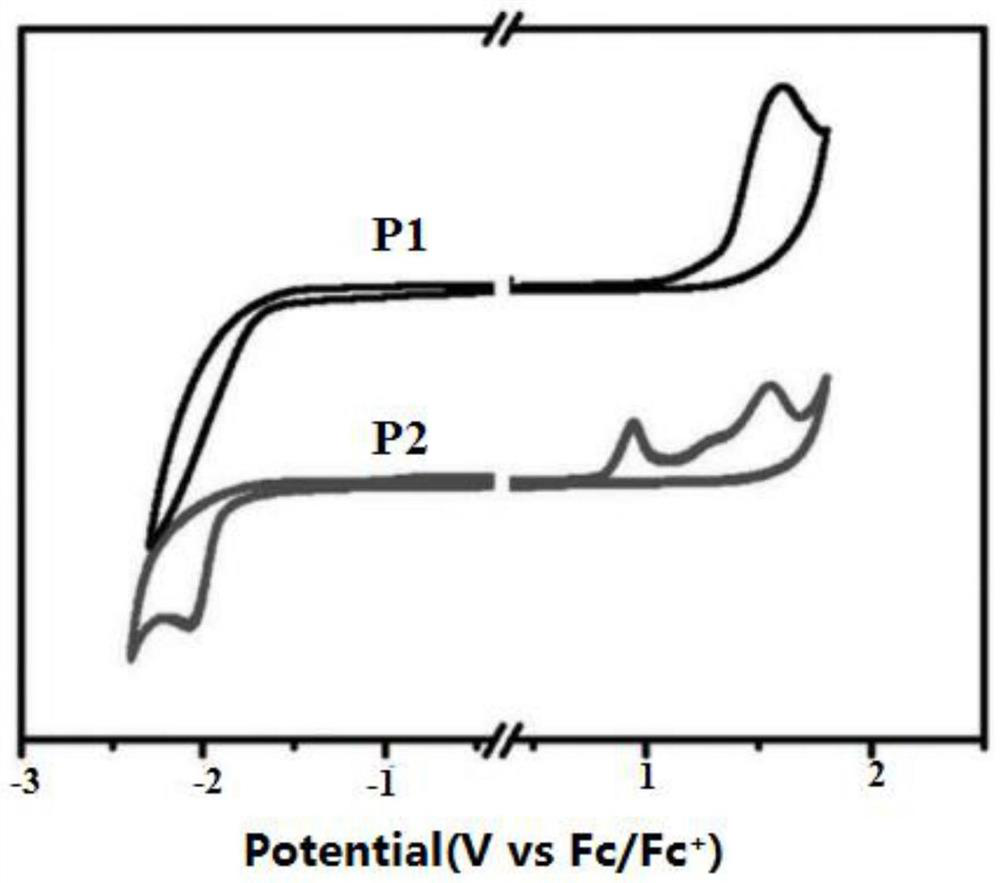
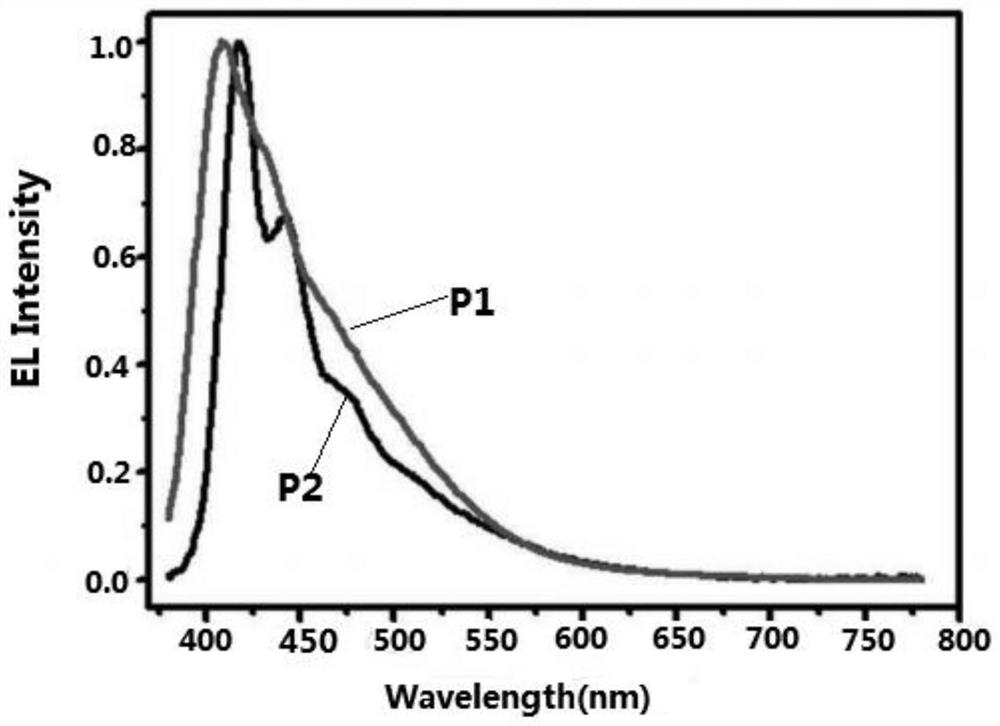
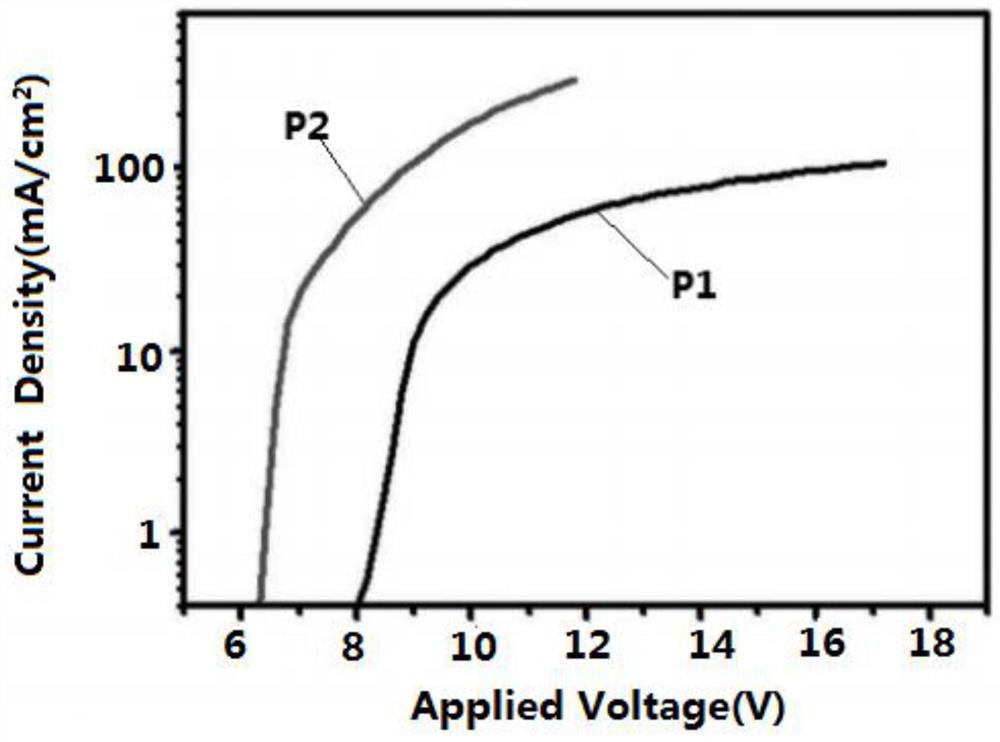
Image
Examples
Embodiment 1
[0021] Under the protection of argon, add 4,4'-diiodobiphenyl (8.12g, 20mmol) and acetic acid (60ml) into a 150ml two-necked flask, and add the solution dissolved in 30ml acetic acid with a constant pressure dropping funnel under ice bath. Bromine (2.31ml, 45mmol), then the reaction solution was raised to room temperature and reacted for 12 hours. After the reaction, the excess liquid bromine was removed with sodium bisulfite aqueous solution, the product was extracted with dichloromethane (100ml), the organic phase was washed 4 times with saturated sodium chloride aqueous solution, dried, filtered, and the solvent was spin-dried. The crude product was petroleum ether. The eluent was subjected to silica gel column chromatography to obtain 2-bromo-4,4'-diiodo-1,1'-biphenyl (7.56g, yield 78%). The chemical reaction equation is:
[0022]
Embodiment 2
[0024] Under the protection of nitrogen, add 3,6-dioctylcarbazole (3.91g, 10mmol), 5ml of triethylamine, and trimethylamine hydrochloride (1.0g, 10mmol) into a 100ml three-necked flask equipped with a constant pressure dropping funnel. And 30ml dichloromethane. Cool to 0°C in an ice bath, add p-toluenesulfonyl chloride (2.8 g, 15 mmol), and continue to react in an ice bath for 2 hours. After the reaction, extract with dichloromethane, wash the organic phase with water and use anhydrous MgSO 4 dry. The solvent was removed by rotary evaporation under reduced pressure, and the crude product was subjected to silica gel column chromatography using petroleum ether / ethyl acetate (volume ratio = 10:1) as the eluent to obtain the compound represented by formula (III) (8.1 g, yield 93.0 %), the chemical reaction equation is:
[0025]
Embodiment 3
[0027] Under the protection of nitrogen, magnesium chips (4.8 g, 20 mmol), the compound represented by formula (III) (8.03 g, 10 mmol) and 50 ml of THF were added to a 250 ml three-necked flask. Then add 2,7-dibromospiro[fluorene-9,2'-oxirane] (3.52g, 10mmol) dissolved in 20ml THF, while slowly heating to 75℃ to initiate the reaction, and the reaction system is refluxed React for 4 hours. Under the protection of nitrogen, ethyl formate (2.4g, 33mmol) and 100ml of THF were added to another 250ml three-necked flask equipped with a constant pressure dropping funnel and reacted at 25°C. The mixed solution after the reaction was transferred to a constant pressure low liquid funnel with a syringe, and 50 ml of ethyl formate solution was added dropwise. After the addition, the reaction solution was allowed to stand for 12 hours, and then an excess of methanol was added to the reaction solution to quench the reaction, and 100ml of saturated ammonium chloride aqueous solution was poure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com