Preparation process of methyl-methyl-3, 4-dihydro-2H-pyran-5-carboxylate
A kind of technology of carboxylate and methyl, applied in the field of preparation technology of methyl-methyl-3,4-dihydro-2H-pyran-5-carboxylate, can solve the problem of expensive raw materials, difficult to obtain, expensive And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
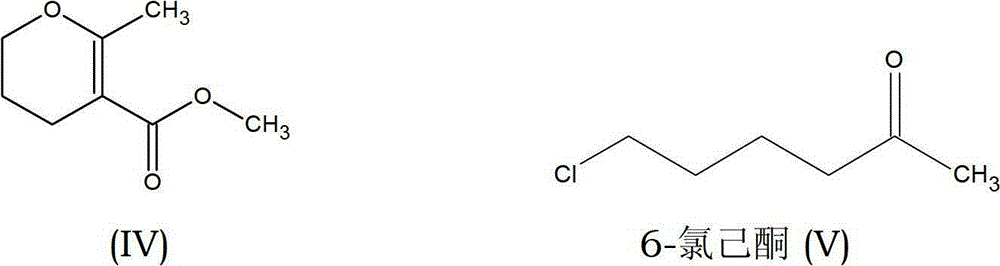
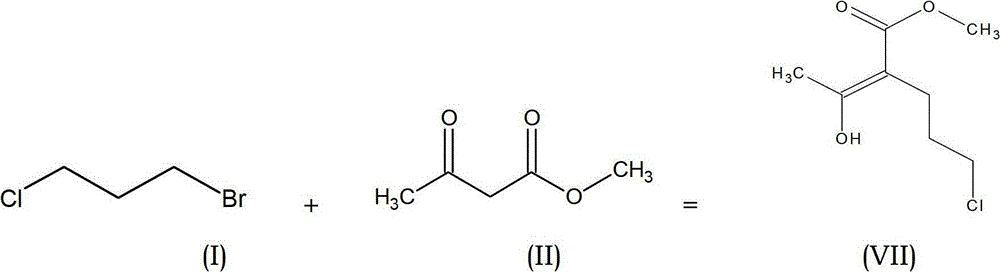
[0048] (1) The preparation of the necessary side chains starts from the condensation of 1-bromo-3-chloropropane of formula (I) with methyl acetoacetate of formula (II) to prepare haloketones of formula (VII).
[0049]
[0050] (2) O-alkylation of haloketones of formula (VII) with sodium methoxide under variable reaction conditions to yield the desired molecule, methyl-methyl- 3,4-Dihydro-2H-pyran-5-carboxylate with simultaneous production of methanol.
[0051]
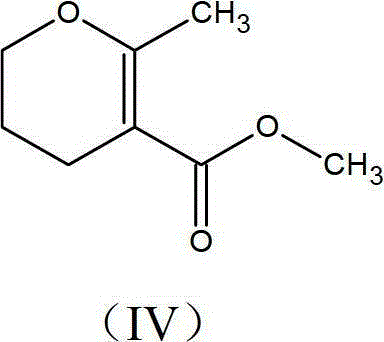
[0052] (3) The crude product is then purified by fractional distillation, methyl-methyl-3,4-dihydro-2H-pyran-5-carboxylate of formula (IV).
[0053]
[0054] In this improved process, the reaction is carried out in the presence of an alcohol solvent selected from the group consisting of methanol, ethanol, isopropanol or n-propanol, pentanol, hexanol and propanol, preferably methanol. The improved process can be carried out with sodium methoxide in solution and powder form, said solution being suitably selecte...
Embodiment -1
[0058] Add 315.0g (2.0mol) of 1-bromo-3-chloropropane and 250.0g (2.15mol) of methyl acetoacetate to 780.0ml of alcohol solvent (preferably methanol) at 30-40°C without maintaining a nitrogen atmosphere . To the above contents, 160.0 g (2.96 mol) of sodium methoxide in the form of powder was slowly added in batches at 30-40°C under a nitrogen atmosphere. Since the reaction was exothermic, a nitrogen atmosphere had to be maintained and the temperature of the reaction mass rose immediately. The reaction is maintained at a reflux temperature of 70°C (limit) for 10-12 hours, preferably 6-8 hours, the reaction mass is cooled to 55-60°C. At this stage approximately 315.0 g of water was added at atmospheric pressure to dissolve any salts formed during the reaction in the water. Fractional distillation of the crude product yields unreacted compound of formula (I) and methanol, the solvent used in the reaction. The solvent methanol recovered by fractional distillation is not in pure...
Embodiment -2
[0060] Add 315.0g (2.0mol) of 1-bromo-3-chloropropane and 250.0g (2.15mol) of methyl acetoacetate to 780.0ml of alcohol solvent (preferably methanol) at 30-40°C without maintaining a nitrogen atmosphere . To the above contents, 184.0 g (3.4 mol) of sodium methoxide in the form of powder was slowly added in portions at 30-40°C under a nitrogen atmosphere. Since the reaction was exothermic, a nitrogen atmosphere had to be maintained and the temperature of the reaction mass rose immediately. The reaction is maintained at a reflux temperature of 70°C (limit) for 10-12 hours, preferably 6-8 hours, the reaction mass is cooled to 55-60°C. At this stage approximately 315.0 g of water was added at atmospheric pressure to dissolve any salts formed during the reaction in the water. Fractional distillation of the crude product yields unreacted compound of formula (I) and methanol, the solvent used in the reaction. The solvent methanol recovered by fractional distillation is not in pure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com