Carbon, silver-copper and polyaniline composite electro-catalyst for oxygen reduction reaction of fuel cell and preparation method and application of electro-catalyst
An electrocatalyst, fuel cell technology, applied in organic compound/hydride/coordination complex catalysts, battery electrodes, chemical instruments and methods, etc., can solve problems such as weak activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
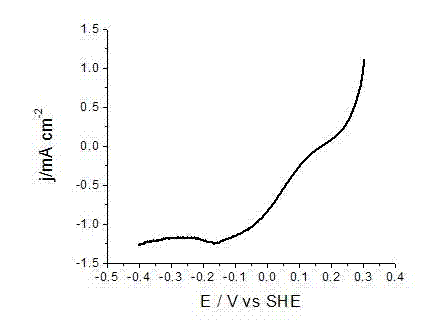
Embodiment 1
[0023] AgNO at a concentration of 8 mM 3 50 mL of ethanol solution, and 2 mM Cu(NO 3 ) 2 ·3H 2 50 mL of O ethanol solution was placed in a hydrothermal reaction kettle, 75 mg of Vulcan XC-72 carbon powder was added, and ultrasonic treatment was performed at room temperature for 5 min; then, 50 mL of 0.5 M NaOH ethanol solution was slowly added to the In the above-mentioned hydrothermal reaction kettle, the addition is completed, and the ultrasonic treatment is performed for 20 minutes; then the reaction kettle is heated to 200 o Keep 0.5 hour after C; After the reaction is completed, the reactor is cooled to room temperature, and the reaction solution is filtered, and the gained nano-catalyst particles are washed twice with pure water and absolute ethanol successively to obtain the carbon-supported silver-copper binary nano-metal catalyst; The carbon-supported silver-copper binary nano-metal catalyst was mixed with 1 ml of 8 mM aniline hydrochloric acid solution, and after ...
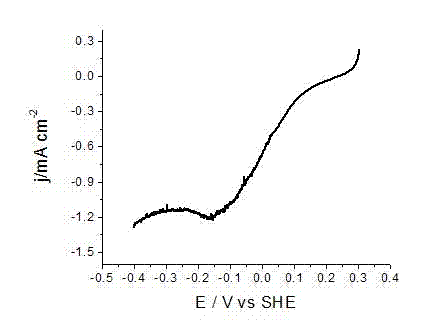
Embodiment 2
[0025] AgNO at a concentration of 8 mM 3 50 mL of ethanol solution, and 8 mM Cu(NO 3 ) 2 ·3H 2 50 mL of O ethanol solution was placed in a hydrothermal reaction kettle, 90 mg of Vulcan XC-72 carbon powder was added, and then ultrasonic treatment was performed at room temperature for 20 min; then, 15 mL of 2 M NaOH ethanol solution was slowly added to the In the above-mentioned hydrothermal reaction kettle, the addition is completed, and the ultrasonic treatment is performed for 20 minutes; then the reaction kettle is heated to 180 o Keep 2 hours after C; After the reaction is completed, the reactor is cooled to room temperature, and the reaction solution is filtered, and the gained nano-catalyst particles are washed twice with pure water and dehydrated alcohol successively to obtain the carbon-supported silver-copper binary nano-metal catalyst; The carbon-supported silver-copper binary nano-metal catalyst was mixed with 10 ml of 2 mM aniline hydrochloric acid solution, and ...
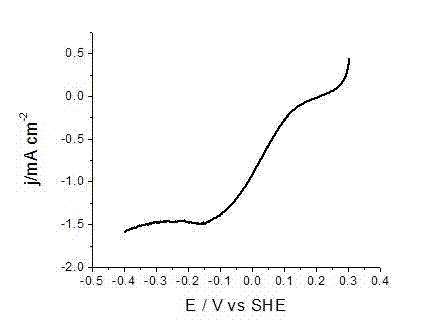
Embodiment 3
[0027] AgNO at a concentration of 6 mM 3 50 mL of ethanol solution, and a concentration of 4 mM Cu(NO 3 ) 2 ·3H 2 50 mL of O ethanol solution was placed in a hydrothermal reaction kettle, 80 mg of Vulcan XC-72 carbon powder was added, and then ultrasonic treatment was carried out at room temperature for 10 min; then, 20 mL of 1 M NaOH ethanol solution was slowly added to the In the above-mentioned hydrothermal reaction kettle, the addition is completed, and the ultrasonic treatment is performed for 10 minutes; then the reaction kettle is heated to 150 o Keep 5 hours after C; After the reaction is completed, the reaction kettle is cooled to room temperature, and the reaction solution is filtered, and the gained nano-catalyst particles are washed twice with pure water and absolute ethanol successively to obtain the carbon-supported silver-copper binary nano-metal catalyst; The carbon-supported silver-copper binary nano-metal catalyst was mixed with 6 ml of 4 mM aniline hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com