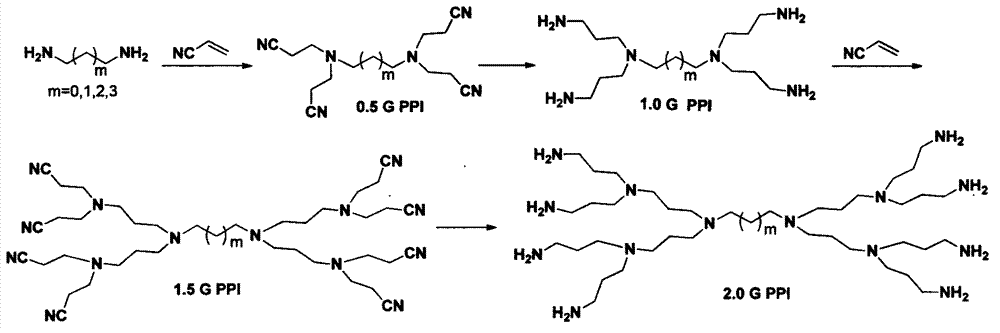
Method of preparing integral generation polypropyleneimine dendrimer by reducing cyano group
A dendrimer and integer technology, applied in the field of reducing cyano groups to prepare primary amine compounds, can solve the problems of side reactions, difficult to obtain, expensive RaneyCo, etc., and achieve the effect of mild reaction conditions and low equipment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The reduction reaction temperature was set to room temperature (25°C).
[0046] Add 2.0g of 0.5 generation PPI, 2.0g of Raney Ni (50μm), and 13ml of methanol into a 250ml double-necked flask equipped with a magnet, and stir at room temperature (25°C) to obtain reaction solution 1; dissolve 1.51g of sodium borohydride in In 16ml of 8M NaOH aqueous solution, the reaction solution 2 was obtained; the reaction solution 2 was added dropwise to the two-necked flask equipped with the reaction solution 1, and the rate of addition was controlled to make the reaction proceed smoothly; after about 3 hours of dropping, stirred at room temperature, TLC (Thin Layer Chromatography, thin-layer chromatography) tracked the reaction until the disappearance of the raw material 0.5 generation PPI took 1h, stopped the reaction, and obtained the reaction solution 3; the reaction solution 3 was subjected to suction filtration to remove Raney Ni, to obtain the filtrate 1, and depressurized Afte...
Embodiment 2
[0048] The reduction reaction temperature was set at 50 °C.
[0049] Add 5.0g of 0.5 generation PPI, 5.12g of Raney Ni (50μm), and 32ml of methanol into a 250ml two-necked flask equipped with a magnet, stir and heat to 50°C under oil bath conditions to obtain reaction solution 1; Dissolve 1 g of sodium borohydride in 40 ml of 8M NaOH aqueous solution to obtain reaction solution 2; add reaction solution 2 dropwise to the double-necked flask containing reaction solution 1, and control the rate of addition to make the reaction proceed smoothly; the addition is completed in about 2 hours Afterwards, stir at 50°C, follow the reaction by TLC until the disappearance of the raw material 0.5-generation PPI takes 1 hour, stop the reaction, and obtain the reaction solution 3; filter the reaction solution 3 to remove Raney Ni, obtain the filtrate 1, and evaporate it under reduced pressure After most of the solvent in filtrate 1, use 100ml of CHCl 3 Extracted 3 times, and concentrated the...
Embodiment 3
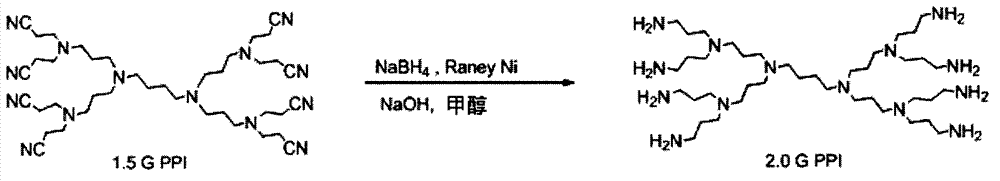
[0052] Set the quality of Raney Ni to 6 times the mass of 1.5G PPI, and the molar number of sodium borohydride to be 3 times the molar number of cyano groups in 1.5G PPI to synthesize 2.0G PPI. The synthetic route is as follows figure 2 shown.
[0053] Add 2.0g of 1.5 generation PPI, 12.0g of Raney Ni (50μm, Aladdin) and 50ml of methanol into a 250ml two-necked flask equipped with a magnet, stir and heat to 70°C under oil bath conditions to obtain reaction solution 1 2.45g sodium borohydride is dissolved in the 8M NaOH aqueous solution of 14ml, obtains reaction solution 2; The reaction solution 2 is added dropwise in the two-necked flask that reaction solution 1 is housed, and the rate of addition is controlled to make the reaction proceed steadily; After stirring at 70°C, react for 3 hours, TLC (Thin Layer Chromatography, thin layer chromatography) tracking reaction shows that the raw material 1.5 generation PPI is exhausted, stop the reduction reaction, cool to room tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com