Pyrazoline derivatives, and preparation method and application thereof
A derivative, pyrazoline technology, applied in the application field of Zn2+ fluorescent probes, can solve the problems of rare ion probes and achieve good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 preparation of pyrazoline derivatives
[0023] Dissolve m-methyl-o-hydroxyacetophenone (1) (12g), o-allyloxybenzaldehyde (2) (13g), sodium hydroxide (48g) in 150ml ethanol, react in ice-water bath for 8h, and judge by TLC After the reaction, add dilute hydrochloric acid solution to neutralize to pH = 7, filter to obtain the crude reaction product, recrystallize with 40ml ethanol, filter and drain to obtain chalcone derivative (3) (18.546g), orange-red crystals, yield 78.8%. The melting point is 166-167°C.
[0024] H NMR spectrum determination: 1 H NMR (300MHz, CDCl 3 ):2.34(s,3H,CH 3 ),4.67(dt,2H,J=5.4,1.5Hz,CH 2 in allyloxy moiety), 5.36 (dd, 1H, J ABcis =9,J BC =1.5Hz,=CHH),5.50(dd,1H,J ACtrans =19,J BC =1.5Hz,=CHH),6.10-6.23(m,1H,=CH-),6.93(d,1H,J=8.4Hz,ArH),6.95(t,1H,J=8.7Hz,ArH),7.31 (d,1H,J=8.4Hz,ArH),7.38(t,1H,J=8.4Hz,ArH),7.65(d,1H,J=8.4Hz,ArH),7.69(s,1H,ArH), 7.87(d,1H,J trans =15.6Hz,=CH-,conjugated vinyl),8.19(d,1H,J trans =15.6Hz,=...
Embodiment 2
[0025] Example 2 Preparation of Pyrazoline Derivatives (4)
[0026] Dissolve compound 3 (5.882g), thiosemicarbazide (2.73g), and sodium hydroxide (2.4g) in 70ml of ethanol, heat and reflux for 4h, after TLC judges that the reaction is over, neutralize the reaction mixture with dilute hydrochloric acid to pH= 7. The crude product was obtained by filtration and recrystallized with 300ml of ethanol to obtain the product pyrazoline derivative (4), a white solid with a yield of 52% and a melting point of 233-234°C.
[0027] Infrared spectrum measurement: IR (KBr), υ: 3436.6, 3326.8, 1600.4, 1481.1, 1337.7, 1250.9, 816.8, 746.7cm -1 .
[0028] H NMR spectrum determination:1 H NMR (300MHz, CDCl 3 ):2.27(s,3H,CH 3 ),3.25(dd,1H,J=18,3.6Hz,CHH in pyrazoline moiety),3.90(dd,1H,J=18,11.4Hz,CHH in pyrazoline moiety),4.57(d,2H,J=5.4Hz ,CH 2 in allyloxy moiety), 5.23 (d, 1H, J ABcis =10.5Hz,=CH),5.37(d,1H,J ACtrans =17.1,=CHH),5.93-6.06(m,1H,=CH-),6.24(dd,1H,J=11.4,3.6Hz,CH in pyrazo...
Embodiment 3
[0033] Embodiment 3 Fluorescence spectrophotometry test
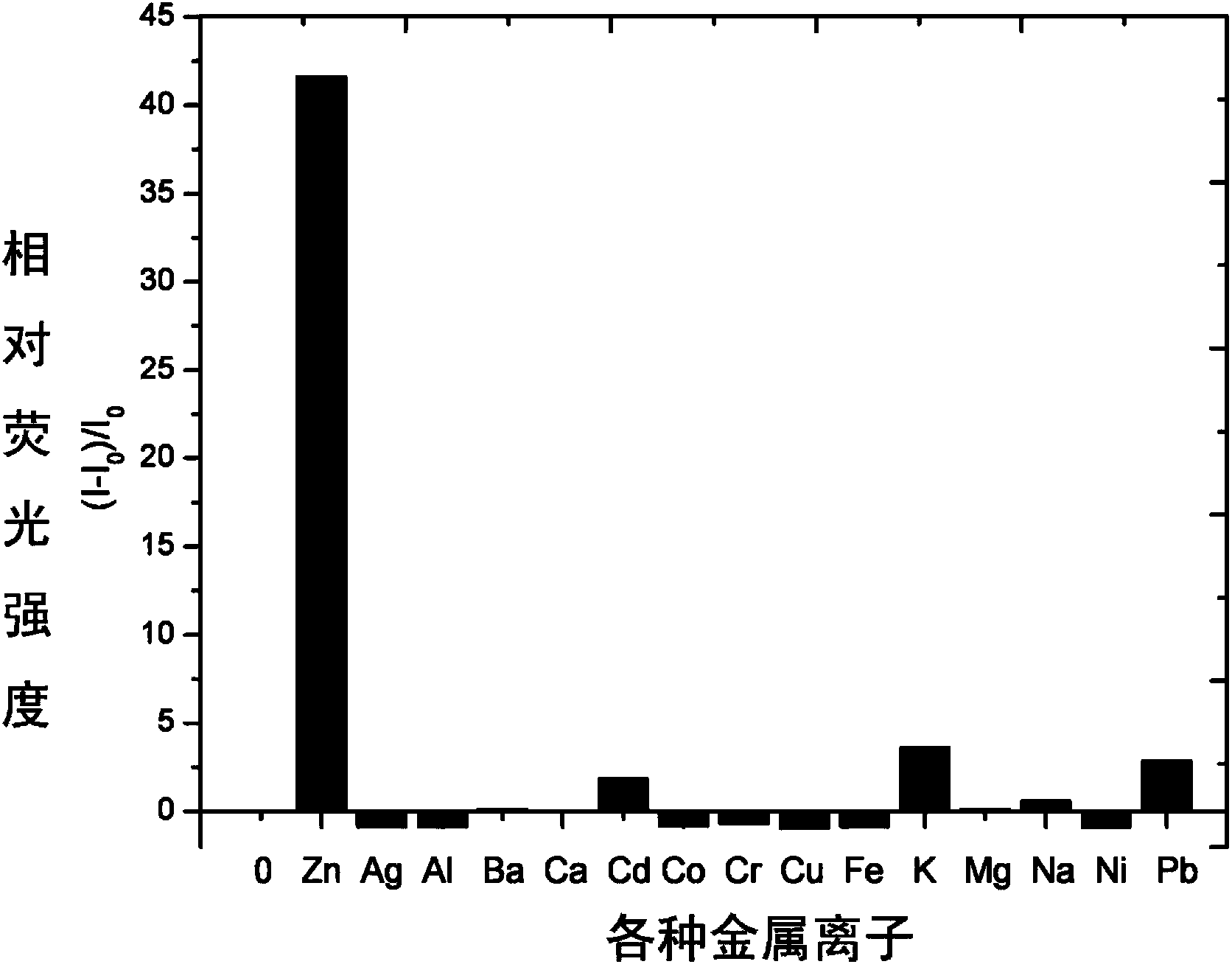
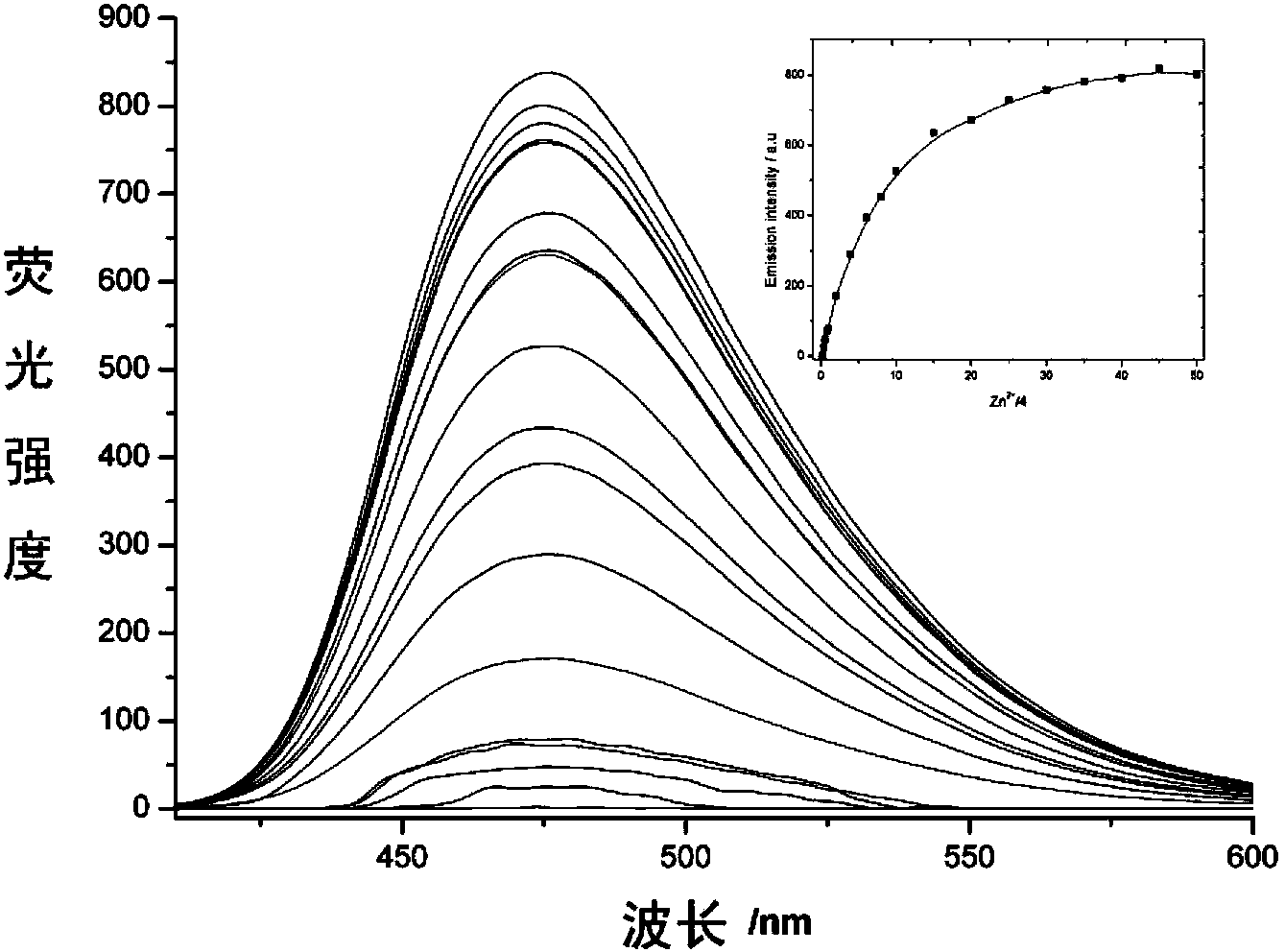
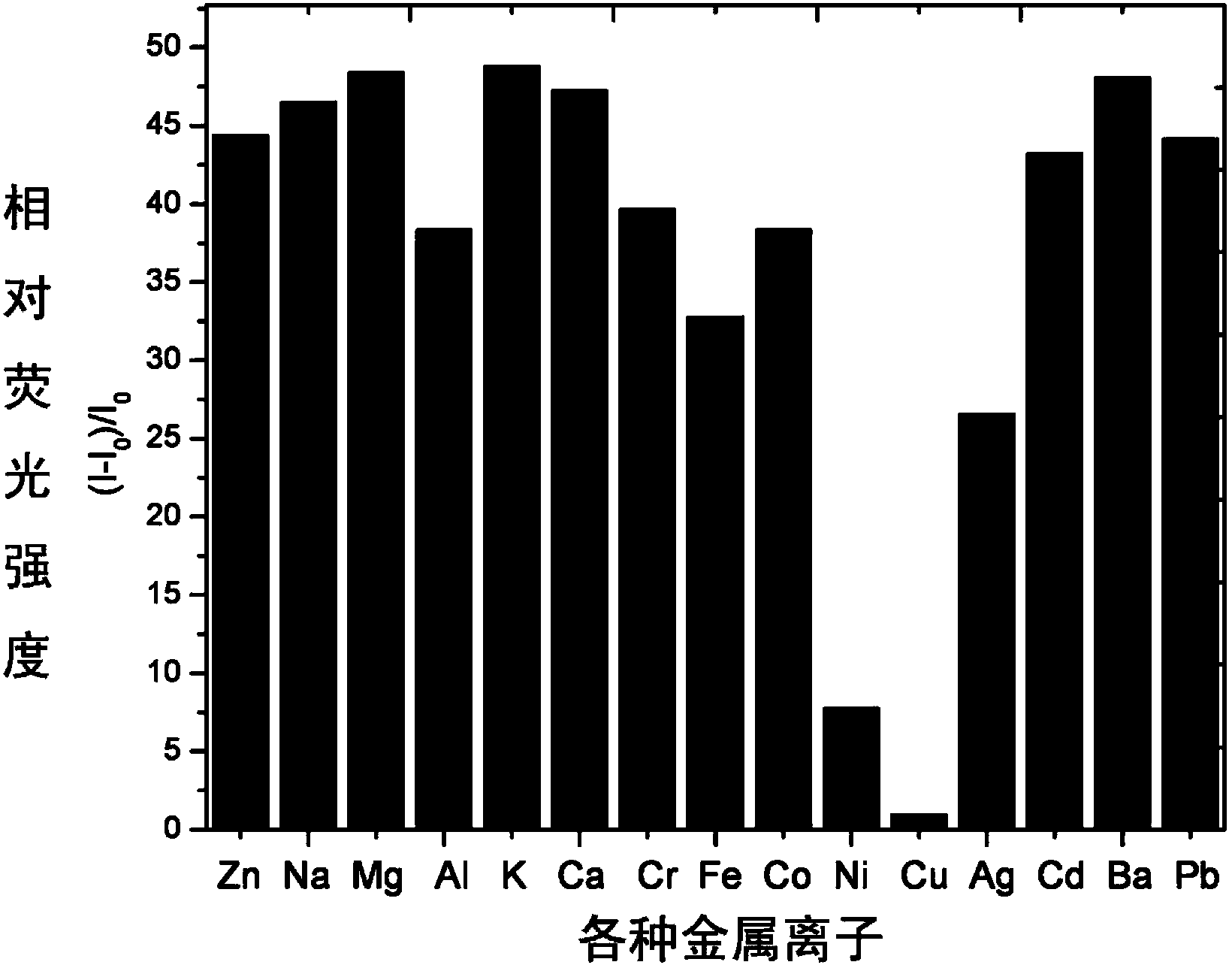
[0034] Use a micro syringe to add 10 equivalents of Na + ,Mg 2+ ,Al 3+ , K + , Ca 2+ ,Cr 3+ ,Mn 2+ , Fe 3+ ,Co 2+ , Ni 2+ ,Zn 2+ , Ag + ,Cd 2+ , Ba 2+ ,Pb 2+ ,Hg 2+ and Cu 2+ Ionic aqueous solutions were tested by fluorescence spectrophotometry.
[0035] The result is as figure 1 , showing that the pyrazoline derivative (4) on Zn 2+ It has very good selectivity, and the contrast before and after adding zinc ions shows that the fluorescence is enhanced by 40 times, and has a strong fluorescence enhancement effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com