Pemetrexed quality control method, and preparation of pemetrexed impurity and salt thereof
A pemetrexed and impurity technology, applied in the field of medicinal chemistry, can solve the problems of lack of reliable basis for drug safety and effectiveness research, difficulty in ensuring product quality, etc., achieve high product purity, strengthen impurity control, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
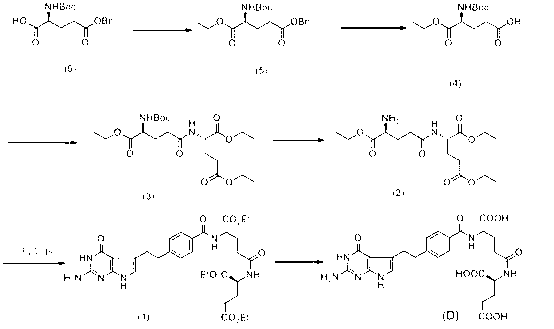
[0031] Dissolve 7.8g of the compound represented by formula (6) in 100ml of dichloromethane solution, then add 4.8g of dicyclohexylcarbodiimide, 0.2g of 4-dimethylaminopyridine, 5ml of absolute ethanol, and stir at room temperature for reaction 4 Hours, the solvent was removed by suction filtration to obtain 8.3 g of the compound represented by formula (5). MS[M+1] + 366.
[0032] Dissolve 8.3 g of the compound represented by formula (5) in 60 ml of methanol, add 0.8 g of palladium carbon, hydrogenate at room temperature for 2 hours, remove the palladium carbon by suction filtration, and remove the solvent from the filtrate under reduced pressure to obtain 4.6 g of the compound represented by formula (4). compound. MS[M-1] - 274.
[0033] Dissolve 4.6g of the compound shown in formula (4) in 50ml of dichloromethane solution, add 7.8g of N,N-dicyclohexylcarbodiimide, 3.3g of hydroxybenzotriazole, 11.5g of diethyl glutamic acid Esters, reacted for 1 hour while stirring at 0...
Embodiment 2
[0044]Dissolve 8.2g of the compound represented by formula (6) in 100ml of ether solution, then add 4.5g of dicyclohexylcarbodiimide, 0.2g of 4-dimethylaminopyridine, 5ml of absolute ethanol, and stir at room temperature for 2 hours. The solvent was removed by suction filtration to obtain 8.3 g of the compound represented by formula (5). MS[M+1] + 366.
[0045] Dissolve 8.2 g of the compound represented by formula (5) in 60 ml of 2-propanol, add 0.8 g of palladium carbon, hydrogenate at room temperature for 1 hour, remove the palladium carbon by suction filtration, and remove the solvent from the filtrate under reduced pressure to obtain 4.5 g of formula (4) Compounds shown. MS[M-1] - 274.
[0046] Dissolve 4.5g of the compound shown in formula (4) in 50ml petroleum ether solution, add 7.8g N,N-dicyclohexylcarbodiimide, 3.3g hydroxybenzotriazole, 4.5g diethyl glutamate , reacted for 1 hour while stirring at 0°C, and reacted for 10 hours while stirring at room temperature,...
Embodiment 3
[0057] Dissolve 7.6g of the compound represented by formula (6) in 100ml of petroleum ether solution, then add 4.5g of dicyclohexylcarbodiimide, 0.2g of 4-dimethylaminopyridine, 5ml of absolute ethanol, and stir at room temperature for 6 hours , and the solvent was removed by suction filtration to obtain 8.3 g of the compound represented by formula (5). MS[M+1] + 366.
[0058] Dissolve 8.0 g of the compound represented by the formula (5) in 60 ml of isobutanol, add 0.8 g of palladium carbon, hydrogenate at room temperature for 3 hours, remove the palladium carbon by suction filtration, and remove the solvent from the filtrate under reduced pressure to obtain 4.5 g of the compound represented by the formula (4). the indicated compounds. MS[M-1] - 274.
[0059] Dissolve 4.5g of the compound shown in formula (4) in 50ml of ether solution, add 7.8g of N,N-dicyclohexylcarbodiimide, 3.3g of hydroxybenzotriazole, 4.5g of diethyl glutamate, React for 1 hour while stirring at 0°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com