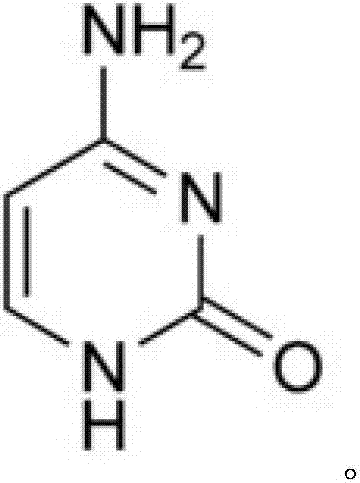
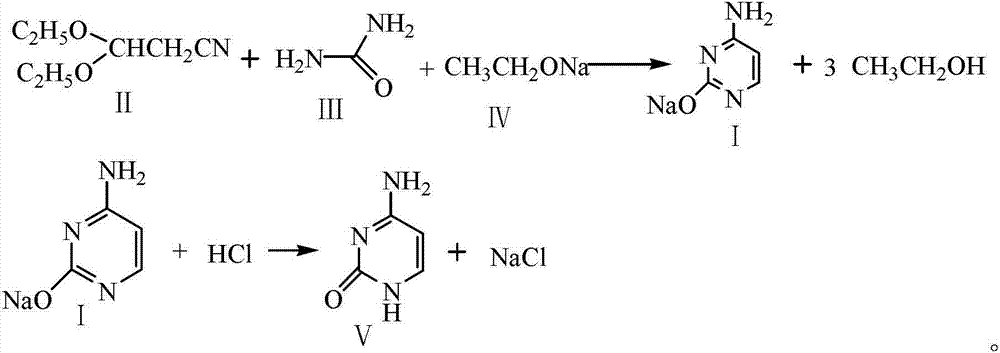
Preparation method for cytosine
A technique of cytosine and dropwise addition, applied in the direction of organic chemistry and the like, can solve problems such as difficult operation and severe reaction, and achieve the effects of low cost, high yield, and cheap and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0037] The preparation of embodiment 13,3-diethoxypropionitrile
[0038] (1) Add 550g (5.97mol) of toluene, 300g (4.05mol) of ethyl formate, 53g (1.29mol) of acetonitrile and 85g (1.25mol) of sodium ethylate into the condensation kettle. After nitrogen replacement, heat to 50°C for condensation reaction for 40h. After the reaction, the temperature was lowered to (31±3)°C to obtain the condensation reaction product.
[0039] (2) Add 500 g of toluene and 200 g of methanol hydrochloride to the acidification kettle in advance (the mass ratio of hydrochloric acid to methanol is 25:75), press the condensation reaction product into the acidification kettle, and the acidification temperature is 18°C. After the acidification was completed, 85 g of sodium hydroxide was added and stirred for 0.5 h to obtain an acidified solution. Suction filter the acidified solution into the concentration kettle. Control the temperature of the concentration tank to rise slowly to a temperature of 65°...
Embodiment 2
[0040] The preparation of embodiment 2 cytosine
[0041] Add 85 g (1.25 mol) of sodium ethylate, 500 g (4.71 mol) of xylene and 111 g (1.85 mol) of urea into the reaction kettle. The reaction kettle was heated to 95°C and stirred for 0.5h. 147.3 g (1.03 mol) of 3,3-diethoxypropionitrile prepared in Example 1 was added dropwise, the temperature was controlled at 100° C., the dropwise addition was completed, and the reaction was refluxed for 8 h. After the reaction was completed and concentrated to dryness, 1200 g of distilled water was added to dissolve the concentrate, and the mixture was allowed to stand to separate layers. The aqueous layer was added dropwise with 5 mol / L hydrochloric acid aqueous solution (0.62 mol hydrochloric acid), stirred for 30 min, and then adjusted to pH=7.0 with aqueous sodium hydroxide solution. Cooled to 0 ° C crystallization 8h. Centrifugation yielded 118 g of crude product.
[0042]Add 1500g of water and 20g of activated carbon into the refi...
Embodiment 3
[0046] The preparation of embodiment 3 cytosine
[0047] Add 85 g (1.25 mol) of sodium ethylate, 550 g (5.18 mol) of xylene and 96 g (1.50 mol) of urea into the reaction kettle. The reaction kettle was heated to 90°C and stirred for 0.3h. 143 g (1.00 mol) of 3,3-diethoxypropionitrile was added dropwise, the temperature was controlled at 105° C., and the reaction was refluxed for 10 h after the dropwise addition was completed. After the reaction was completed and concentrated to dryness, 1200 g of distilled water was added to dissolve the concentrate, and the mixture was allowed to stand to separate layers. Concentrated 5 mol / L hydrochloric acid aqueous solution (0.8 mol hydrochloric acid) was added dropwise to the aqueous layer, stirred for 30 min, and then adjusted to pH=7.5 with aqueous sodium hydroxide solution. Cooled to -5 ° C crystallization 8h. Centrifugation yielded 115 g of crude product.
[0048] Add 1500g of water and 10g of activated carbon into the refining ke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com