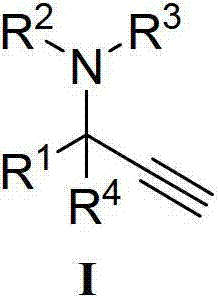
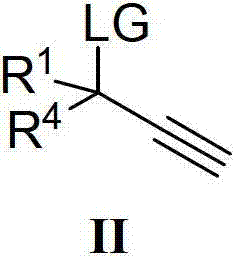
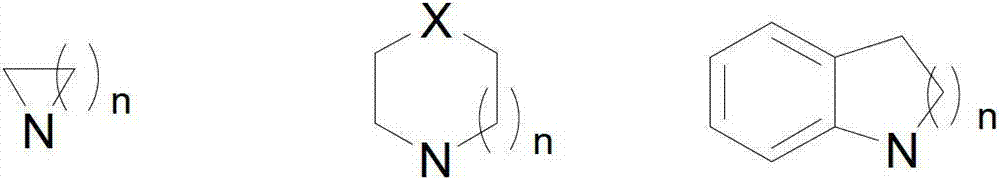Method for catalytic synthesis of chiral propargylamine compound by chiral copper catalyst
A technology of chiral copper catalyst and propargyl amine is applied in the preparation of amino compounds, the preparation of organic compounds, the preparation of amino hydroxyl compounds, etc. small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] Add metal precursor CuCl (0.015mmol, 5mol%) and chiral ligand L-3-1 (0.0165mmol, 5.5mol%) into the reaction flask, add 1 ml of anhydrous methanol under nitrogen protection, and stir at room temperature for 1 hour. Dissolve propargyl alcohol ester II-1 (0.3 mmol, 1 equiv), amine III-1 (0.36 mmol 1.2 equiv) and N,N-diisopropylethylamine (0.36 mmol 1.2 equiv) in 1 ml of anhydrous methanol, Then the solution was added into the above catalyst solution under the protection of nitrogen, and the reaction was stirred at room temperature for 12 hours. After the reaction was completed, rotary evaporation under reduced pressure and column separation gave colorless oily liquid I-1 (57.0 mg, 86% yield). The optical purity of I-1 by HPLC analysis was 95%ee.HPLC conditions: chiralcel OD-H, 40°C, 254nm, n-hexane / 2-propanol=95 / 5, flow rate=0.8mLmin -1 , major enantiomer: t 1 =5.59min;minor enantiomer:t 2 =6.37min.[α] D 20 =-11(c 0.7, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ...
Embodiment 2
[0051] II-1 in Example 1 is replaced by II-2, and all the other conditions are the same as in Example 1. To obtain 66.6mg of colorless oily liquid, 87% yield, 96%ee. HPLC conditions: chiralcel OD-H, 40°C, 254nm, n-hexane / 2-propanol=95 / 5, flow rate=0.8mLmin -1 , major enantiomer: t 1 =6.36min;minor enantiomer:t 2 =7.16min.[α] D 20 =+18(c 0.4,CHCl 3 ); 1 HNMR (400MHz, CDCl 3 ):δ=7.80(d,1H),7.42(d,1H),7.41-7.24(m,4H),7.06(d,2H),6.87(t,1H),5.98(s,1H),2.69( s,3H),2.51(s,1H)ppm; 13 C NMR (100MHz, CDCl 3 ):δ=149.7,135.1134.5,130.6,130.1,129.5,129.1,126.6,119.2,115.6,79.3,75.3,54.5,33.3ppm; HRMS calcd.for C 16 h 15 ClN[M+H]: 256.0893, found: 256.0893.
[0052]
Embodiment 3
[0054] II-1 in the embodiment is replaced by II-3, and all the other conditions are the same as in Example 1. 65.8 mg of colorless oily liquid was obtained, with a yield of 86%, and 97% ee. HPLC conditions: chiralcel OD-H, 40°C, 254nm, n-hexane / 2-propanol=95 / 5, flow rate=0.8mL / min, major enantiomer:t 1 =7.29min;minor enantiomer:t 2 =8.00min.[α] D 20 =4(c 0.5,CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ):δ=7.60(s,1H),7.47-7.49(m,1H),7.33-7.29(m,4H),6.99-7.01(m,2H),6.87-6.91(m,1H),5.74(s ,1H),2.72(s,3H),2.57(s,1H); 13 C NMR (100MHz, CDCl 3 ):δ=149.8,140.1,134.5,129.7,129.2,128.0,127.6,125.6,119.3,115.5,79.1,75.4,56.2,33.8; HRMS calcd.for C 16 h 15 ClN[M+H]: 256.0893, found: 256.0891.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com