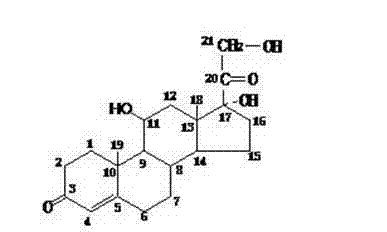
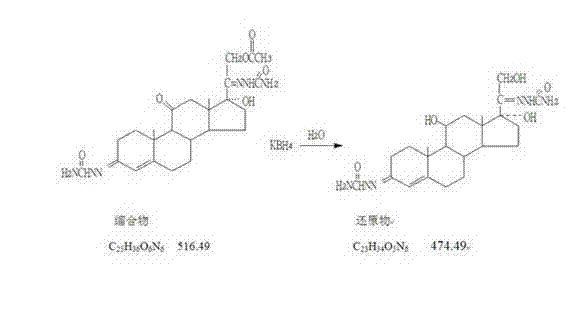
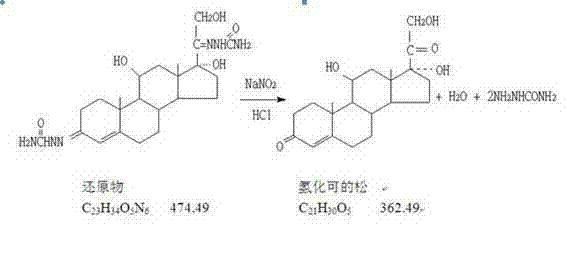
Process for preparing hydrocortisone
A technology for hydrocortisone and preparation process, which is applied in the production of organic chemistry, steroids, and bulk chemicals, etc., can solve the problems of low chromatographic purity of the target product, high risk of impurity generation, increased production cost, etc., and achieves improved quality. and yield, shorten the production cycle, reduce the effect of labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0015] Dissolve 10g of sodium nitrite in 100ml of drinking water, stir to dissolve, set aside, add 10ml of dichloromethane to 400ml of 5% hydrochloric acid solution, stir evenly, add 20g of hydrocortisone reducer, stir to dissolve at 30-35°C Clear, slowly add sodium nitrite solution dropwise, finish dropping in 3-4 hours, continue to react for 2 hours, take samples for testing, after passing the test, cool down to 0-5°C to filter and discharge, vacuum dry, dry to obtain hydrocortisone The crude hydrolyzate was 14.30g, the yield increased by 6.21%, and the chromatographic purity: 96.5%.
Embodiment 2
[0017] Dissolve 10g of sodium nitrite in 100ml of drinking water, stir to dissolve, set aside, add 10ml of dichloromethane to 450ml of 5% hydrochloric acid solution, stir evenly, add 20g of hydrocortisone reducer, stir to dissolve at 30-35°C Clear, slowly add sodium nitrite solution dropwise, finish dropping in 3-4 hours, continue to react for 2 hours, take samples for testing, after passing the test, cool down to 0-5°C to filter and discharge, vacuum dry, dry to obtain hydrocortisone The hydrolyzate crude product was 14.36g, the yield increased by 6.53%, and the chromatographic purity: 96.0%.
Embodiment 3
[0019] Dissolve 10g of sodium nitrite in 100ml of drinking water, stir to dissolve, set aside, add 10ml of dichloromethane to 350ml of 5% hydrochloric acid solution, stir evenly, add 20g of hydrocortisone reducer, stir to dissolve at 30-35°C Clear, slowly add sodium nitrite solution dropwise, finish dropping in 3-4 hours, continue to react for 2 hours, take samples for testing, after passing the test, cool down to 0-5°C to filter and discharge, vacuum dry, dry to obtain hydrocortisone The crude hydrolyzate was 14.27g, the yield increased by 6.08%, and the chromatographic purity: 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com