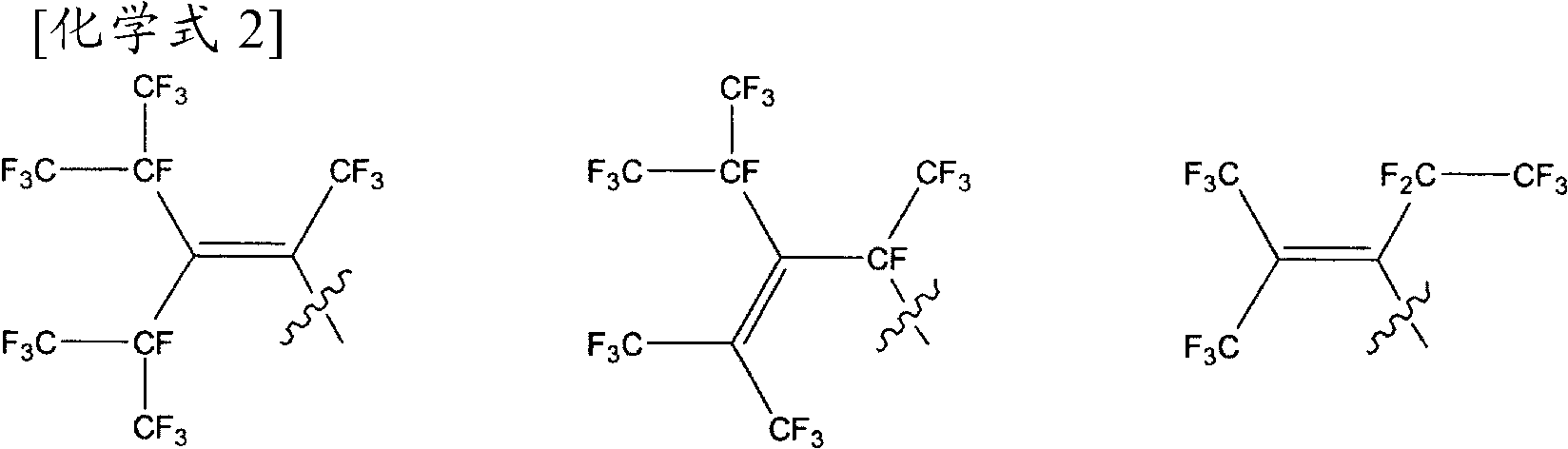
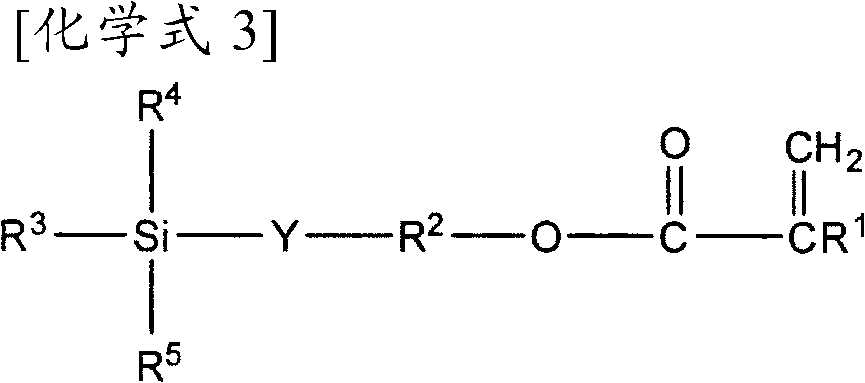
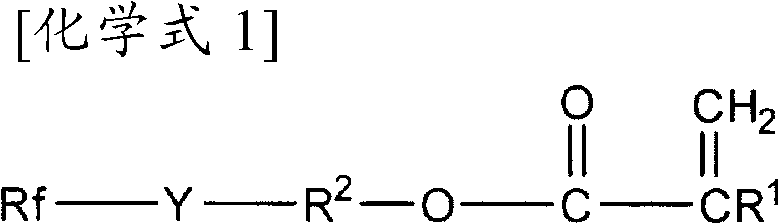Agent for imparting antifouling properties
A technology of imparting agent and antifouling property, applied in the field of new fluorine-containing siloxane copolymer, can solve the problems of reduced film strength, disappearance of hydrophobic and oleophobic effect, and inability to obtain easily, and achieves improved solubility, improved film strength, The effect of good surface modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0127] The content of the present invention is described by the following examples, but it should not be interpreted that the content of the present invention is limited by the examples.
Synthetic example 1
[0129] Into a three-necked flask (3 L) equipped with a dropping funnel, 259.5 g (1.8 mol) of 4-hydroxybutyl acrylate, 218.6 g (2.16 mol) of triethylamine, and 1,000 g of acetonitrile were added. 973.0 g (2.16 mol) of tripolyhexafluoropropylene was added to the dropping funnel, and slowly added dropwise to the solution in the flask over about 60 minutes while stirring. After completion of the dropwise addition, stirring was further continued at room temperature for 3 hours.
[0130] 2200 g of 1N hydrochloric acid was added to the reaction mixture to terminate the reaction, and the reaction mixture was transferred to a 5 L beaker, and washed with 1 L of water three times. The solution after the water washing treatment was dehydrated under reduced pressure to obtain 964.0 g of fluorinated acrylate represented by the following formula (A-1) (yield 93%). The obtained fluorinated acrylate (A-1) 1 H-NMR data are shown in Table 1.
[0131] [chemical formula 13]
[0132] RfO(CH 2 ...
Synthetic example 2
[0141] In a three-necked flask (100 mL) equipped with a cooling tube, 2.87 g (5 mmol) of the fluorine-containing acrylate (A-1) synthesized in Synthesis Example 1, 2.11 g of SILAPLANE TM-0701T (B-1) manufactured by Chisso Corporation, g (5mmol), 5.12g (10mmol) of BLEMMER AE-400 (C-1) manufactured by NOF Corporation, 10.10g (10mmol) of propylene glycol monomethyl ether acetate, 0.10g (0.6mmol) of 2,2'-azobisisobutyronitrile ), lauryl mercaptan 0.27g (1.3mmol). Nitrogen gas was introduced into the reaction solution, and the inside of the reaction container was replaced with nitrogen. After nitrogen substitution, the reaction solution was heated to 90° C. while stirring the reaction solution to start the reaction. Then, stirring was continued at 90° C. for 21 hours. The reaction ends with 1 It was confirmed by the disappearance of the peaks peculiar to each acrylate in H-NMR. The target fluorine-containing silicone copolymer can be obtained quantitatively (50 mass % propylene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com