Method for preparing lenalidomide
A technology of lenalidomide and nitro group, applied in the field of preparing lenalidomide, can solve the problems of loss of lenalidomide components, time-consuming and laborious, influence on the total yield, etc., and achieve mild reaction conditions, improve the total yield, The effect of efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
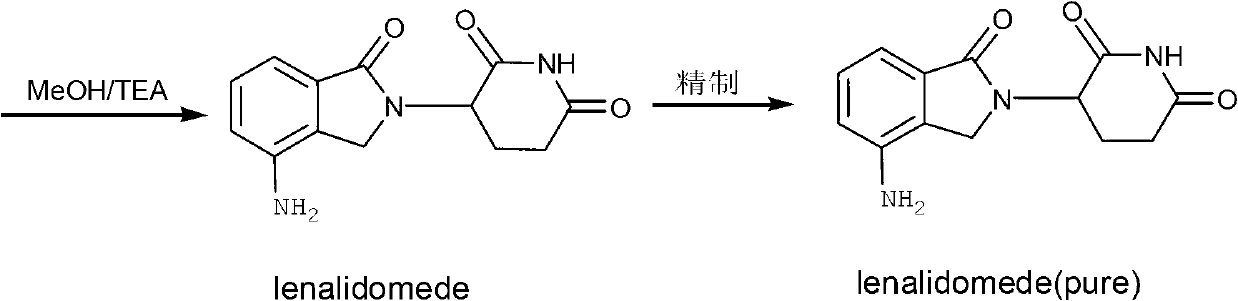
[0031] Embodiment 1: the method of the present invention prepares lenalidomide
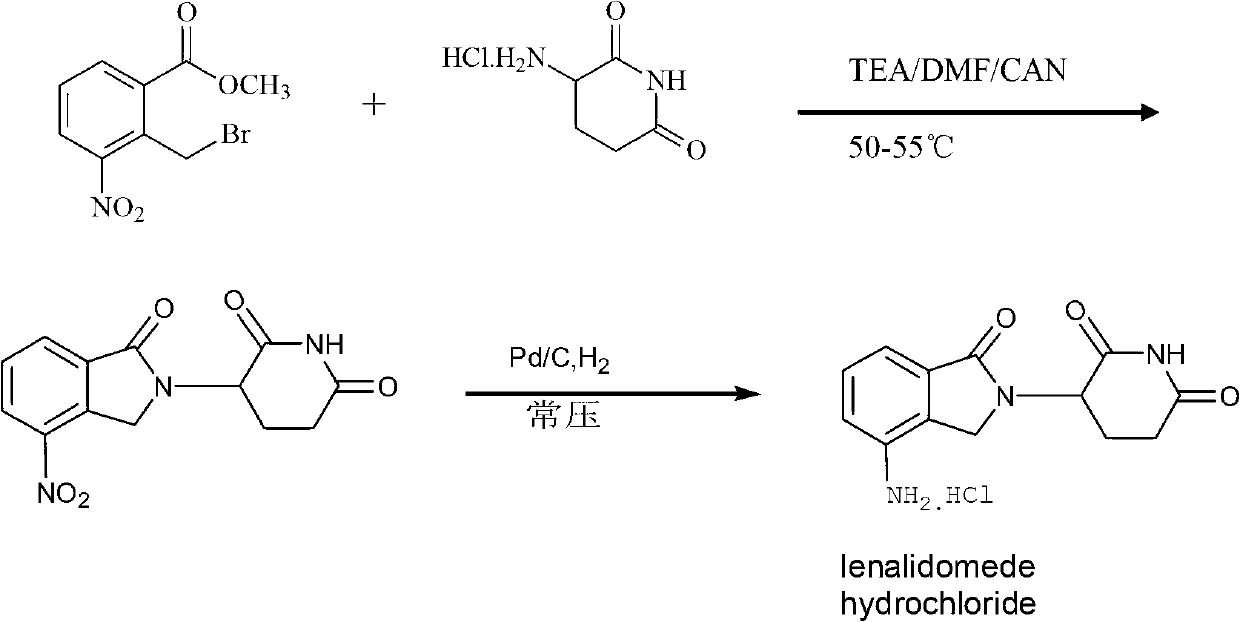
[0032] 1. Preparation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione
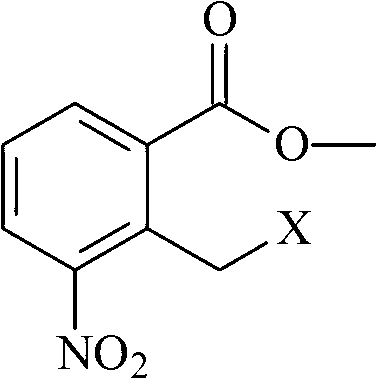
[0033] Add 20.0g of 3-aminopiperidine-2,6-dione hydrochloride and 160ml of DMF to a 500ml single-necked bottle, stir at room temperature for 5 minutes, then add 40ml of triethylamine to the system, stir for 5 minutes, then add 37.2g of 2-bromo Methyl-3-nitro-benzoic acid methyl ester, 40ml of acetonitrile, after addition, under nitrogen protection, stir the reaction at 53°C (the reaction temperature is between 20°C and the boiling point of the mixed solvent of DMF and acetonitrile) for 8 hours, then cool to room temperature Then pour it into 500ml water (2 times the volume of the reaction solution) under stirring, filter after stirring for 10 minutes, rinse and filter with 500ml water (2 times the volume of the reaction solution) and 500ml methanol (2 times the volume of the reaction solution) respectively. Cake, th...
Embodiment 2
[0042] Embodiment 2: the method of the present invention prepares lenalidomide
[0043] 1. Preparation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione
[0044] Add 20.0g of 3-aminopiperidine-2,6-dione hydrochloride and 300ml of acetonitrile into a 500ml single-necked bottle, stir at room temperature for 30 minutes, then add 40ml of triethylamine to the system, stir for 30 minutes, then add 37.2g of 2- Bromomethyl-3-nitro-methyl benzoate, after addition, stirred and reacted at 81°C (reaction temperature between 20°C and solvent boiling point) under nitrogen protection for 12 hours, cooled to room temperature and poured into 3000ml of water (9 times the volume of the reaction solution), stirred for 10 minutes and then filtered, followed by rinsing the filter cake with 3000ml of water (9 times the volume of the reaction solution) and 3000ml isopropanol (9 times the volume of the reaction solution). The cake was dried under reduced pressure at 50°C to obtain a blue-purp...
Embodiment 3
[0053] Embodiment 3: the method of the present invention prepares lenalidomide
[0054] 1. Preparation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione
[0055] Add 20.0g of 3-aminopiperidine-2,6-dione hydrochloride and 200ml of DMF to a 500ml single-necked bottle, stir at room temperature for 5 minutes, then add 40ml of triethylamine to the system, stir for 5 minutes, then add 37.2g of 2-bromo After the addition of methyl-3-nitro-benzoic acid methyl ester, under nitrogen protection, stir the reaction at 60°C (the reaction temperature is between 20°C and the boiling point of the solvent) for 10 hours, and pour it into 1250ml under stirring after cooling to room temperature water (5 times the volume of the reaction solution), stirred for 10 minutes and then filtered, then rinsed the filter cake with 1250ml water (5 times the volume of the reaction solution) and 1250ml ethanol (5 times the volume of the reaction solution) respectively, and collected the filter cake in 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com