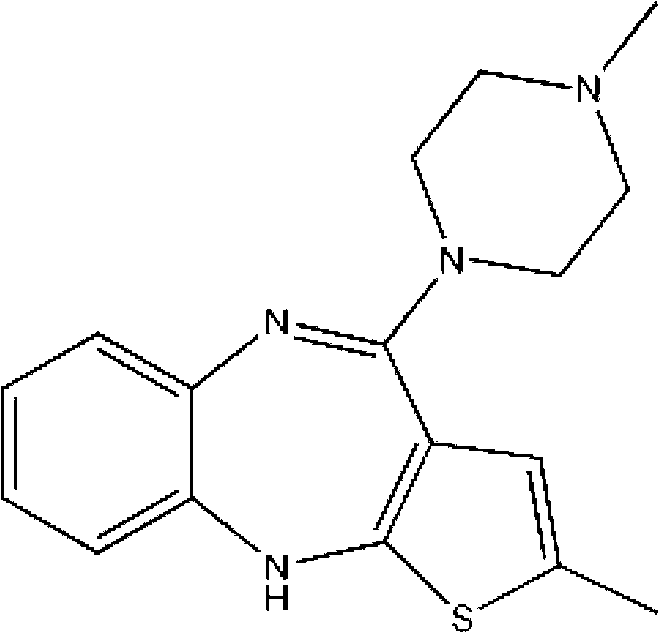
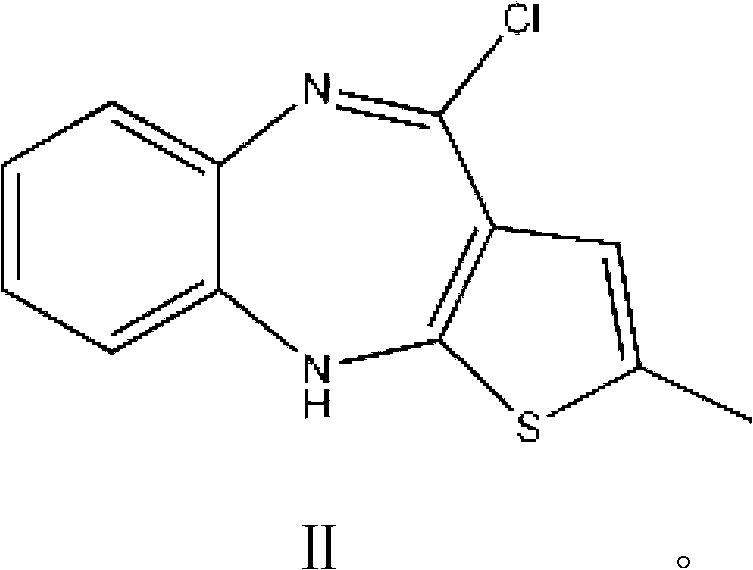
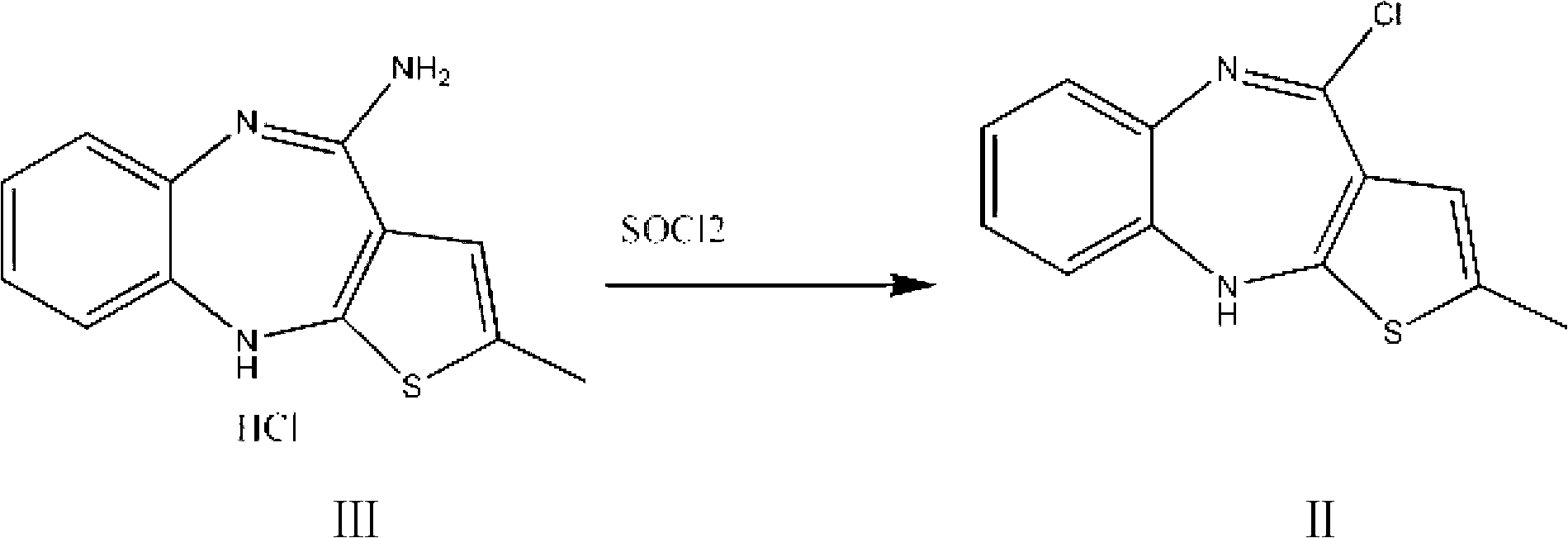
Olanzapine intermediate and method for preparing olanzapine using same
An intermediate, the technology of olanzapine, which is applied in the field of preparation of olanzapine, can solve problems such as difficult removal, difficult handling, and low yield, and achieve the effects of avoiding waste, reducing costs, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The intermediate of olanzapine: 2-methyl-4-chloro-10H-thieno[2,3-b][1,5]benzodiazepine Synthesis:
[0025] Fully dissolve 100g of 2-methyl-4-amino-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride in 500ml of chloroform, and then add 40ml of Thionyl chloride was heated to 60°C and reacted for 6 hours. After all the raw materials were reacted, chloroform and excess thionyl chloride were distilled off under reduced pressure to obtain 108g of olanzapine intermediate: 2-methyl -4-Chloro-10H-thieno[2,3-b][1,5]benzodiazepine.
Embodiment 2
[0027] The synthesis of olanzapine: 2-methyl-4-chloro-10H-thieno[2,3-b][1,5]benzodiazepine 100g was dissolved in 400ml dimethyl sulfoxide and 400ml toluene In the mixed solution, add 54 g of N-methylpiperazine under stirring condition, raise the temperature to 120° C. and react for 8 hours. After all the raw materials have been reacted, toluene and dimethyl sulfoxide are removed by distillation under reduced pressure, and the resulting residual solution is cooled to room temperature. Then pour into 2000ml of ice water to precipitate the solid, filter and wash the resulting solid with ice water, and dry to obtain olanzapine: 2-methyl-4-[4-methyl-1-piperazinyl]-10H-thieno [2,3-b][1,5]benzodiazepines.
Embodiment 3
[0029] Synthesis of olanzapine: 2-methyl-4-chloro-10H-thieno[2,3-b][1,5]benzodiazepine 65g was dissolved in 390ml dimethyl sulfoxide and 270ml toluene Add 14.3 g of N-methylpiperazine to the mixture under stirring condition, raise the temperature to 60°C and react for 16 hours, after all the raw materials have been reacted, distill off toluene and dimethyl sulfoxide under reduced pressure, and cool the obtained residue to room temperature , and then poured into 1000ml of ice water to precipitate the solid, filtered and washed with ice water, and dried to obtain olanzapine: 2-methyl-4-[4-methyl-1-piperazinyl]-10H-thiophene And[2,3-b][1,5]benzodiazepines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com