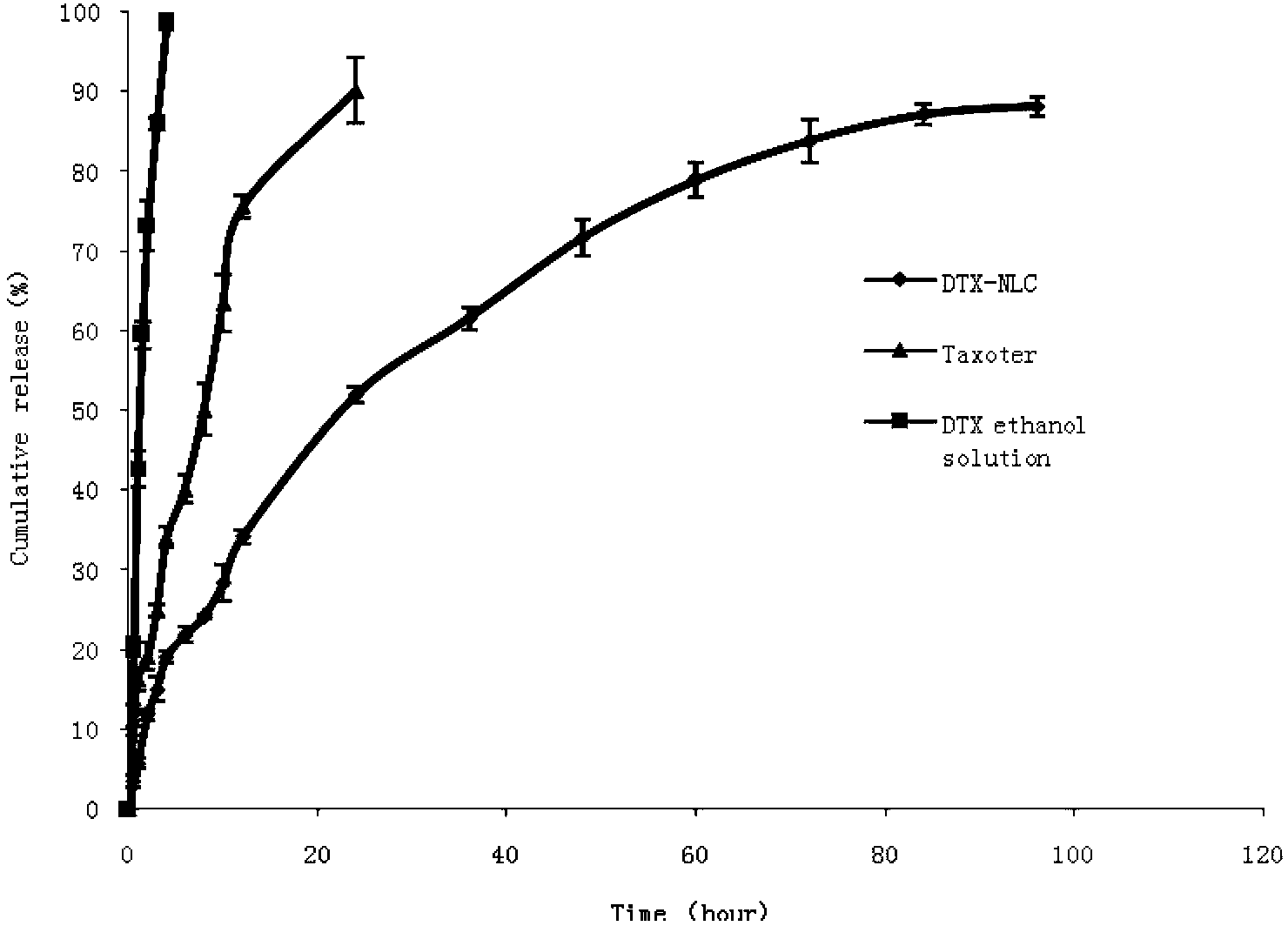
Preparation and Application of Docetaxel Lipid Nanoparticles
A technology of lipid nanoparticles and docetaxel, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, powder delivery, etc., can solve the problems of difficult clinical use, low solubility, non-absorption, etc., and achieve prolonged circulatory retention Time and material cost are low, and the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
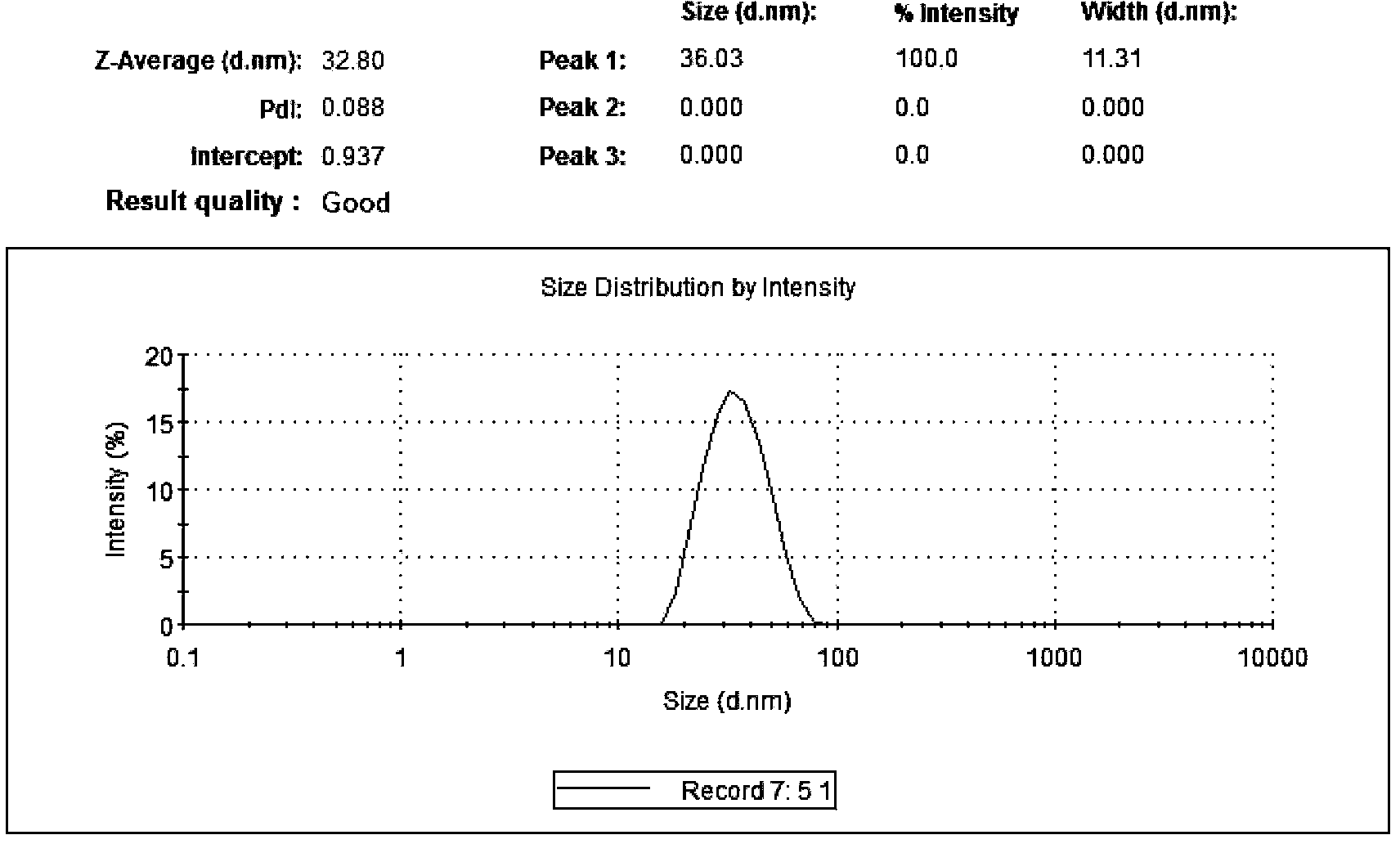
[0059] Weigh 87.5mg cetyl palmitate, 37.5mg caprylic capric triglyceride, 5mg docetaxel, add a small amount of absolute ethanol to dissolve, heat and melt at (60±2)°C, and use it as the oil phase. Weigh 60 mg of soybean lecithin and 90 mg of polyethylene glycol 15 hydroxystearate, add 5 mL of physiological saline for injection to dissolve it ultrasonically, and rapidly heat to the same temperature as the oil phase as the water phase. Under magnetic stirring at 400r / min, the water phase was added dropwise to the oil phase where the ethanol was evaporated while hot, to prepare O / W type colostrum. The prepared colostrum was rapidly dispersed with a probe-type ultrasonic cell pulverizer, with a power of 100W, ultrasonication for 1s, interval of 1s, ultrasonication time for 4min, cooling in an ice-water bath, and passing through a 0.22μm microporous membrane. The measured particle size is 33.83nm, the PDI is 0.089, and the encapsulation efficiency is 98.26%.
example 2
[0061] Weigh 120mg glyceryl behenate, 30mg caprylic capric triglyceride, 5mg docetaxel, add a small amount of methanol to dissolve, heat and melt at (80±2)°C, and use it as the oil phase. Weigh 50 mg of soybean lecithin and 90 mg of polyethylene glycol 15 hydroxystearate, add 5 mL of physiological saline for injection to dissolve it ultrasonically, and rapidly heat to the same temperature as the oil phase as the water phase. Under magnetic stirring at 500r / min, the water phase was added dropwise to the oil phase where the methanol was evaporated while hot to prepare O / W colostrum. The prepared colostrum was quickly dispersed with a probe-type ultrasonic cell pulverizer, with a power of 200W, ultrasonication for 1s, interval of 1s, ultrasonication time for 5min, cooling in an ice-water bath, and passing through a 0.45μm microporous membrane. The measured particle size is 182.52 nm, the PDI is 0.052, and the encapsulation efficiency is 97.59%.
example 3
[0063] Weigh 120mg glyceryl palmitostearate, 30mg caprylic capric triglyceride, 6mg docetaxel, add a small amount of acetone to dissolve, heat and melt at (70±2)°C, and use it as the oil phase. Weigh 50 mg of soybean lecithin, 150 mg of polyethylene glycol 15 hydroxystearate, add 5 mL of physiological saline for ultrasonic dissolution, and rapidly heat to the same temperature as the oil phase as the water phase. Under magnetic stirring at 500r / min, the water phase was added dropwise to the oil phase evaporated from the acetone while hot to prepare O / W colostrum. The prepared colostrum was quickly dispersed with a probe-type ultrasonic cell pulverizer, with a power of 140W, ultrasonic for 1s, intermittent for 1s, ultrasonic time for 7min, cooled in an ice-water bath, and passed through a 0.22μm microporous membrane. The measured particle size is 131.6nm, the PDI is 0.211, and the encapsulation efficiency is 96.50%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com