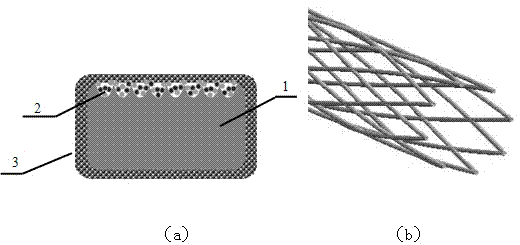
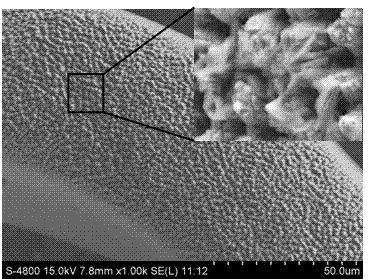
Composite medicine carrying vessel stent and preparation method thereof
A technology of vascular stents and metal stents, applied in the field of vascular stents, which can solve the problems of peeling and falling off of the drug-loaded layer, incomplete drug coverage on the surface of the stent, and poor binding force between the drug-loaded layer and the matrix, so as to prevent the occurrence of thrombus and restenosis , promote the effect of vascular endothelialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In the first step, the preparation of the drug-loaded solution on the surface of the vascular stent The ratio of the components of the drug-loaded solution is:
[0041] Drug-loaded solution components
concentration
VEGF
50mg / L
Na 2 HPO 4
5g / L
tris hydroxymethyl amino methane
6g / L
5g / L
[0042] The loaded drug solution is adjusted to a pH value of 5 with dilute hydrochloric acid with a concentration of 10% by weight and ammonia water with a concentration of 10% by weight;
[0043] In the second step, a femtosecond laser pattern is processed on the surface of the vascular stent, and HA and VEGF are co-deposited to form a HA-loaded VEGF drug layer
[0044] Take a container with openings on one side, the width of which is 2cm larger than the diameter of the stent, the length is 2cm longer than the length of the stent, and the height is 0.5cm larger than the diameter of the stent, ...
Embodiment 2
[0052] The first step, the preparation of drug solution loaded on the surface of vascular stent
[0053] The ratio of the loaded drug solution components is:
[0054] Drug-loaded solution components
concentration
VEGF
100mg / L
Na 2 HPO 4
1g / L
tris hydroxymethyl amino methane
10g / L
2g / L
Nano-hydroxyapatite
5g / L
[0055] The loaded drug solution is adjusted to a pH value of 4 with dilute hydrochloric acid with a concentration of 10% by weight and ammonia water with a concentration of 10% by weight;
[0056] In the second step, a femtosecond laser pattern is processed on the surface of the vascular stent, and HA and VEGF are co-deposited to form a HA-loaded VEGF drug layer
[0057] Take a container with openings on one side, the width of which is 2cm larger than the diameter of the stent, the length is 2cm longer than the length of the stent, and the height is 0.5cm larger th...
Embodiment 3
[0064] The first step, the preparation of drug solution loaded on the surface of vascular stent
[0065] The ratio of the loaded drug solution components is:
[0066] Drug-loaded solution components
[0067] The loaded drug solution is adjusted to a pH value of 6 with dilute hydrochloric acid with a concentration of 10% by weight and ammonia water with a concentration of 10% by weight;
[0068] In the second step, a femtosecond laser pattern is processed on the surface of the vascular stent, and HA and VEGF are co-deposited to form a HA-loaded VEGF drug layer
[0069] Take a container with openings on one side, the width of which is 2cm larger than the diameter of the stent, the length is 2cm longer than the length of the stent, and the height is 0.5cm larger than the diameter of the stent, and another container with a diameter smaller than the inner diameter of the stent by 0.1mm but just enough to pass through the above The rotating shaft of the opening on one sid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com