Quality control method of intestine astringe antidiarrheic preparation
A quality control method and technology of pharmaceutical preparations, applied in the direction of weighing by removing certain components, material analysis and measuring devices by observing the impact on chemical indicators, etc., can solve the problems of quantitative control of active ingredients in preparations, sensitivity And the accuracy is weak, the quality control method is too simple, etc., to achieve the effect of a reliable determination method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
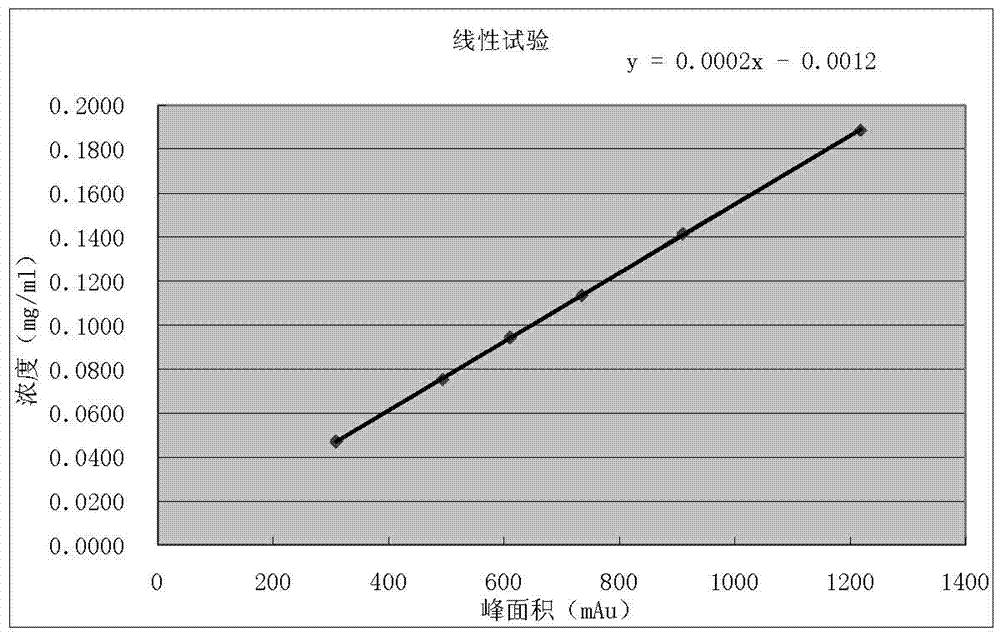
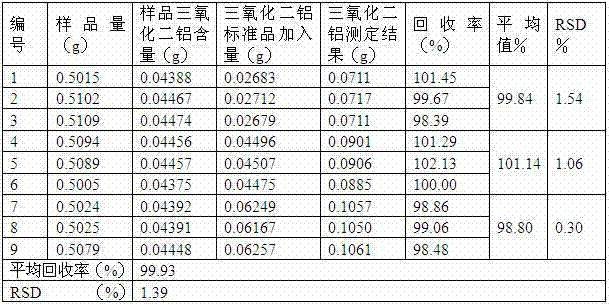
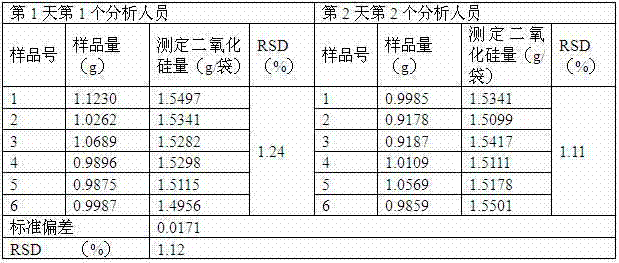
[0040] A, high performance liquid chromatography is used to measure the content of petracenin in this preparation: take octadecyl bonded silica gel as filler; Be mobile phase with the methanol-water solution of 0.2:0.8 with the volume ratio; Detection wavelength is 275nm; The number of plates should not be less than 2000 according to the petracenin peak calculation; the preparation of the reference substance solution: take the petracenin reference substance, accurately weighed, add 99.5% methanol to make a solution containing 0.05mg per 1ml; the test solution Preparation: Take 0.5g of the content of the powder, and accurately weigh it as 0.5010 g , into a 50ml measuring bottle, add 99.5% methanol for ultrasonic treatment for 9min, let it cool, add 99.5% methanol to the mark, shake well, filter through a 0.40μm nylon filter membrane, and take the subsequent filtrate as the test solution; determination method: respectively Precisely draw 5 μl of each of the reference substance s...
Embodiment 2
[0044] A, high performance liquid chromatography is used to measure the content of petracenin in this preparation: take octadecyl bonded silica gel as filler; Be mobile phase with the methanol-water solution of 0.3:0.7 with the volume ratio; Detection wavelength is 275nm; The plate number should not be less than 2000 according to the petracenin peak calculation; the preparation of the reference substance solution: take the petracenin reference substance, weigh it accurately, add 99.0% methanol to make a solution containing 0.15mg per 1ml; the test solution Preparation: Take 1.0g of the tablet and finely grind the content, accurately weigh it as 1.0002g, put it into a 50ml measuring bottle, add 99.0% methanol for ultrasonic treatment for 11min, let it cool, add 99.0% methanol to the mark, shake well, pass through a 0.45μm polymer Tetrafluoroethylene filter membrane is filtered, and the subsequent filtrate is taken as need testing solution; Determination method: each 10 μ l of re...
Embodiment 3
[0048] A, high performance liquid chromatography measures the content of petracenin in this preparation: take octadecyl bonded silica gel as filler; Be mobile phase with the methanol-water solution of 0.25.:075 with volume ratio; Detection wavelength is 275nm; The number of theoretical plates should not be less than 2000 based on the petracenin peak; preparation of the reference substance solution: take an appropriate amount of the petracenin reference substance, weigh it accurately, add 99.9% methanol to make a solution containing 0.1mg per 1ml; the test substance Preparation of the solution: Take 1.5g of the contents in the capsule, accurately weigh 1.4999g, put it into a 50ml measuring bottle, add 99.9% methanol for ultrasonic treatment for 10min, let it cool, add 99.9% methanol to the mark, shake well, pass through 0.50 μm Filtrate with a nylon filter, and take the subsequent filtrate as the test solution; assay method: respectively accurately draw 8 μl of the reference sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com