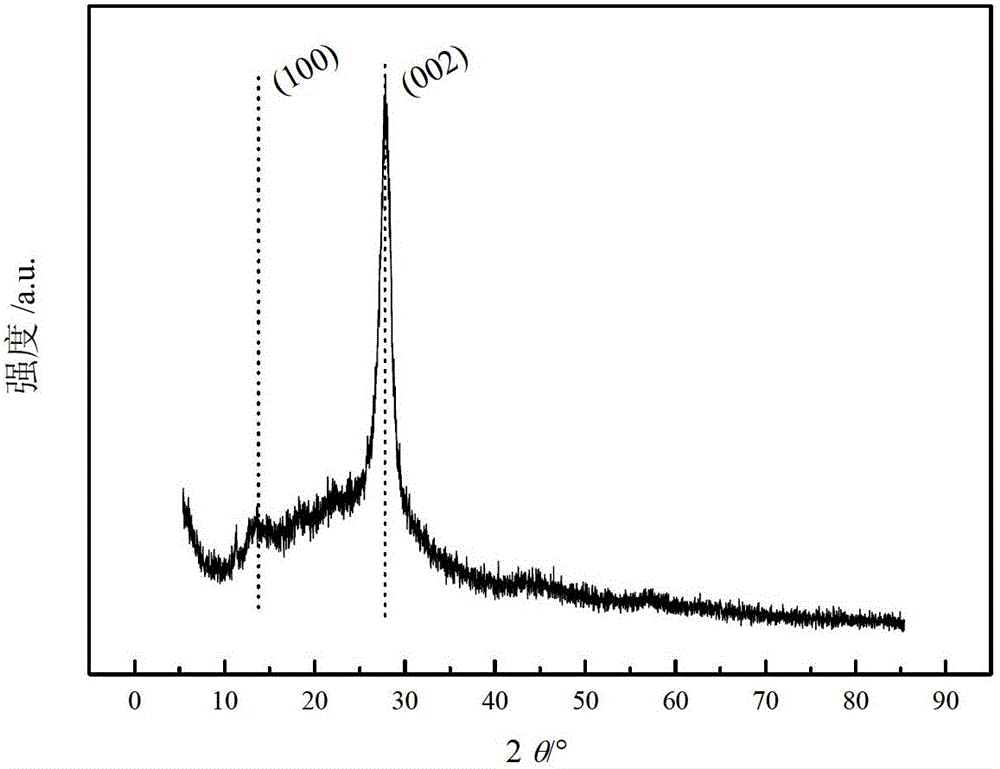
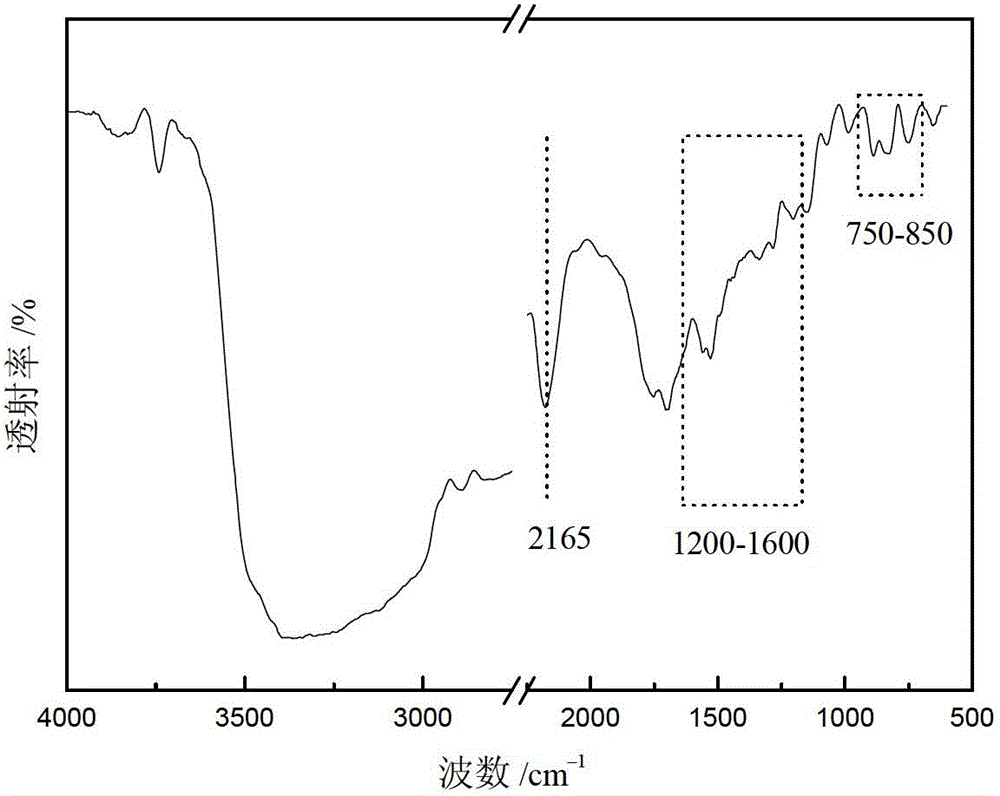
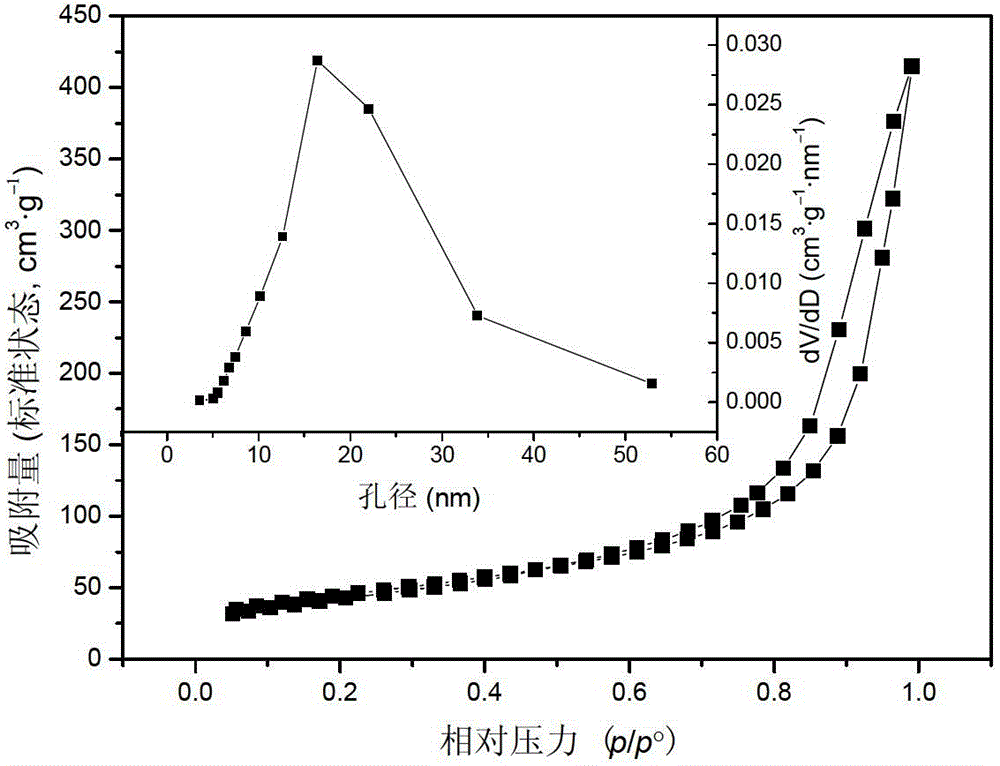
High-specific-surface-area mesoporous graphite-phase carbon nitride material and preparation method thereof
A graphite phase carbon nitride, high specific surface technology, applied in the direction of nitrogen and non-metallic compounds, can solve the problems of small size, difficult to control surface area and pore volume, and highly toxic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve 4 g of guanidine hydrochloride in 6.4 g of water to obtain an aqueous solution of guanidine hydrochloride;
[0029] (2) Drop the guanidine hydrochloride aqueous solution obtained in step (1) into 3.2 g of SiO 2 nanosphere (15nm) powder, and stirred for 3h, forming a white paste solid;
[0030] (3) Dry the white paste solid obtained in step (2) in an oven at 80°C for 8 hours to obtain a white powder;
[0031] (4) Put the above-mentioned white powder obtained in step (3) into a tube furnace with a nitrogen atmosphere and roast it. The temperature programming condition is: from room temperature to 550°C at a rate of 3°C / min, and then keep at this temperature for 2h , yellow powder was obtained after cooling down;
[0032] (5) Disperse the yellow powder obtained in step (4) in 100g NH 4 HF 2 in aqueous solution (solute 24g), stirred for 2 days to remove SiO in the yellow powder 2 ;
[0033] (6) Filtrating or centrifuging the mixture obtained in step (5) t...
Embodiment 2
[0037] (1) Dissolve 4 g of guanidine hydrochloride in 9.6 g of water to obtain an aqueous solution of guanidine hydrochloride;
[0038] (2) Add the guanidine hydrochloride solution obtained in step (1) to 4.8 g of SiO2 at a rate of 2 s per drop. 2 nanosphere (15nm) powder, and stirred for 3h, forming a white paste solid;
[0039] (3) Dry the white paste solid obtained in step (2) in an oven at 100°C for 4 hours to obtain a white powder;
[0040] (4) The white powder obtained in step (3) was roasted in a tube furnace with a nitrogen atmosphere. The temperature programming conditions were as follows: from room temperature to 450 °C at a rate of 3 °C / min, and then kept at this temperature for 4 hours. After cooling down, a yellow powder is obtained;
[0041] (5) Disperse the yellow powder obtained in step (4) in 200g HF aqueous solution (solute 20g), stir for 1 day to remove SiO in the yellow powder 2 ;
[0042] (6) Filtrating or centrifuging the mixture obtained in step (5) ...
Embodiment 3
[0046] (1) Dissolve 4 g of guanidine hydrochloride in 12 g of water to obtain an aqueous solution of guanidine hydrochloride;
[0047] (2) Add the guanidine hydrochloride solution obtained in step (1) dropwise to 10 g of SiO at a rate of 2 s per drop 2 nanosphere (30nm) powder, and stirred for 3h, forming a white paste solid;
[0048] (3) Dry the white paste solid obtained in step (2) in an oven at 100°C for 4 hours to obtain a white powder;
[0049] (4) The white powder obtained in step (3) was roasted in a tube furnace with a nitrogen atmosphere. The temperature programming conditions were as follows: from room temperature to 500 °C at a rate of 3 °C / min, and then kept at this temperature for 3 hours. After cooling down, a yellow powder is obtained;
[0050] (5) Disperse the yellow powder obtained in step (4) in an aqueous solution of 120g HF (solute 12g), and stir for 2 days to remove SiO in the yellow powder 2 ;
[0051] (6) Filtrating or centrifuging the mixture obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com