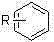Method for synthesizing chlorinated aromatic hydrocarbon with participation of trichloroisocyanuric acid
A technology of trichloroisocyanuric acid and chlorinated aromatic hydrocarbons, which is applied to the chlorination reaction of benzene ring and the synthesis field of chlorinated aromatic hydrocarbon compounds, can solve the problems of low reaction selectivity, high pollutant discharge, unsafe operation and the like, and achieves the The effect of improving economic efficiency, effectively utilizing resources, and being convenient to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In a three-necked flask equipped with a magnetic stirring bar and a reflux condenser, add 1.08 g (10 mmol) of anisole (wherein the R group is CH 3 -), 2.79g (12mmol) trichloroisocyanuric acid, 0.032g (0.4mmol) ammonium nitrate, 30mL acetonitrile, heated up to 85°C, reacted for 1h, stopped the reaction, cooled to room temperature, filtered to remove the solid, and removed with a rotary evaporator After solvent acetonitrile, 1.35 g of colorless oily liquid 4-chloroanisole was obtained, yield: 95%. Boiling point: 199-201°C (101KPa) (literature value: 199-201°C (101KPa)). After testing, the physical property data of the product are all close to the literature values.
[0052] After NMR detection, proton spectrum data were obtained to characterize the structure of the product. The specific data are as follows:
[0053] 1 H NMR (CDCl 3 , 500 MHz) δ: 3.84 (s, 3H, OCH 3 ), 6.81 (d, J =7.6 Hz, 2H, ArH), 7.27 (d, J =7.6 Hz, 2H, ArH).
Embodiment 2
[0055] In a three-necked flask equipped with a magnetic stirring bar and a reflux condenser, add 1.70 g (10 mmol) of diphenyl ether (where the R group is C 6 h 5 O-), 2.79g (12 mmol) trichloroisocyanuric acid, 0.032g (0.4 mmol) ammonium nitrate, 30mL acetonitrile, heat up to 85°C, react for 1h, stop the reaction, cool to room temperature, filter to remove solids, and use rotary evaporation After removing the solvent acetonitrile, 1.96 g of colorless oily liquid 4-chlorodiphenyl ether was obtained. Yield: 96%. Boiling point: 283-285°C (101KPa) (literature value: 283-285°C (101KPa))
[0056] After NMR detection, proton spectrum data were obtained to characterize the structure of the product. The specific data are as follows:
[0057] 1 H NMR (CDCl 3 , 500 MHz) δ: 6.90 (d, J =8.0 Hz, 2H, ArH), 6.99-7.05 (m, 3H, ArH), 7.30-7.33 (m, 2H, ArH), 7.43 (d, J =8.0 Hz, 2H, ArH).
Embodiment 3
[0059] In a three-necked flask equipped with a magnetic stirring bar and a reflux condenser, add 1.23 g (10 mmol) of nitrobenzene (wherein the R group is NO 2 -), 2.79g (12mmol) trichloroisocyanuric acid, 0.81g (5mmol) ferric chloride, 30mL acetonitrile, heat up to 85°C, react for 24h, stop the reaction, and cool to room temperature. The solid was removed by filtration, and 1.42 g of solid 3-chloronitrobenzene was obtained after the solvent was spin-off acetonitrile. Yield: 90%. Melting point: 44-45°C (literature value: 46°C)
[0060] After NMR detection, proton spectrum data were obtained to characterize the structure of the product. The specific data are as follows:
[0061] 1 H NMR (CDCl 3 , 500 MHz) δ: 7.53-7.55 (m, 1H, ArH), 7.68-7.70 (m, 1H, ArH), 8.12-8.14 (m, 1H, ArH), 8.23-8.25 (m, 1H, ArH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com