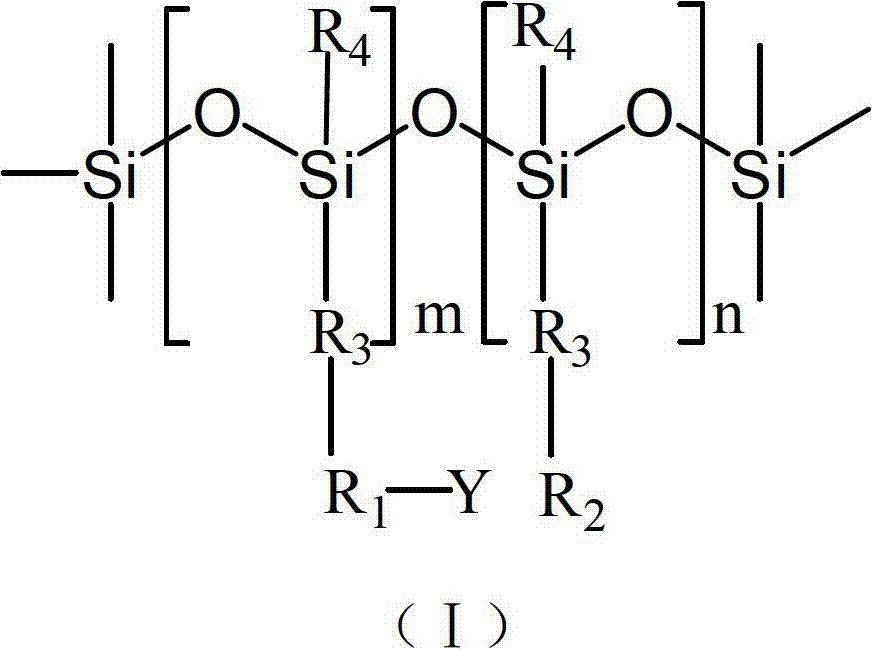
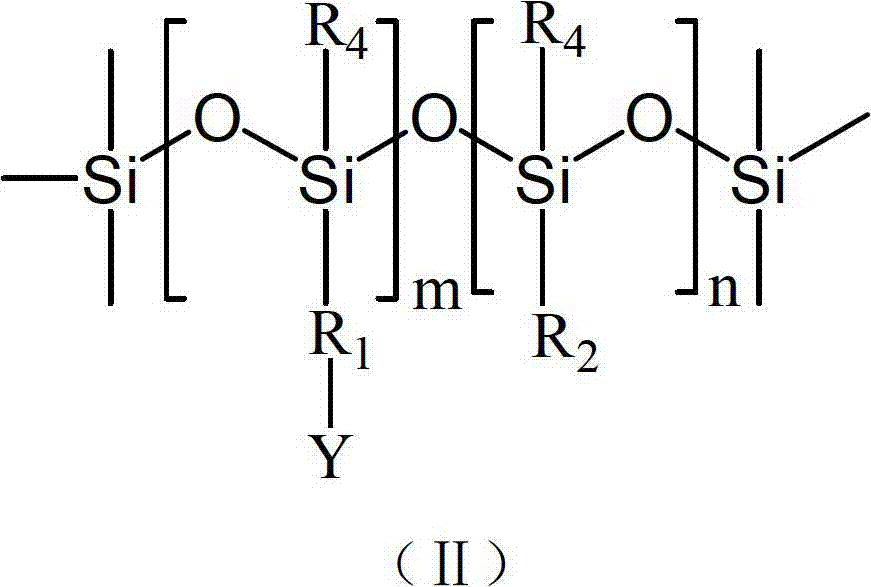
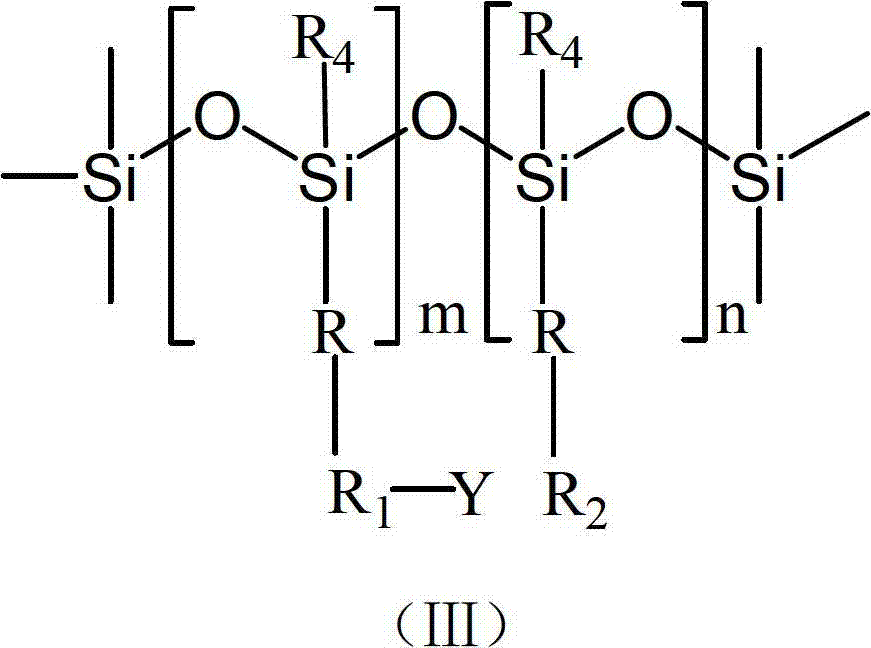
Polysiloxane, preparation method, application and bacteriacide containing same
A polysiloxane and alkyl technology, applied in the field of polysiloxane and its preparation, can solve the problems of poor water solubility of organic heterocyclic structures, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] (1) Magnetic stirring device, add 100mL of absolute ethanol to a 250mL three-necked reaction flask, add 72.14g (0.3mol, 1eq) of chloropropylmethyldimethoxysilane and 0.45g of capping agent hexamethylsiloxane Alkanes, 77.8g of concentrated hydrochloric acid was slowly added dropwise to the reaction liquid, and the reaction was refluxed overnight, and the reaction was completed in about 16 hours. Most of the solvent was removed by rotary evaporation with ethanol and water, and dried in a vacuum oven at 80°C for about 15 hours to obtain a colorless transparent liquid chloropropyl polymethylsiloxane for future use.
[0109] (2) R 1 Preparation of potassium salt: magnetic stirring device, 100mL of absolute ethanol, 25.6g of 5,5-dimethylhydantoin (0.2mol, 1eq) were added to a 250mL three-neck reaction flask, and 11.2g of potassium hydroxide ( 0.2mol, 1eq), stirred at room temperature to a colorless clear solution, and the reaction was completed in about 1h. Most of the solv...
Embodiment 2
[0143] (1) Refer to the following literature (Xing Xin, Li Xiaodong, Guo Aiqing, Cao Feng and Wang Jun, Research on the synthesis of polymethylchlorosilane by chlorination reaction, Journal of Functional Polymers, 2004, 17 (2): 181-184) to carry out polymerization Preparation of Methylchlorosilanes.
[0144] (2) R 1 Preparation of sodium salt: Magnetic stirring device, 100 mL of absolute ethanol, 25.6 g of 5,5-dimethylhydantoin (0.2 mol, 1 eq) were added to a 250 mL three-necked reaction flask, and 8.0 g of sodium hydroxide ( 0.2mol, 1eq), stirred at room temperature to a colorless clear solution, and the reaction was completed in about 1h. Most of the solvents ethanol and water were evaporated by rotary evaporation, and dried in a vacuum oven at 80°C for 4 days to obtain white solid 5,5-dimethylhydantoin sodium salt for future use.
[0145] (3) Magnetic stirring device, add 6g of polymethylchlorosilane and 50mL of anhydrous N,N-dimethylformamide (DMF) into a 100mL three-nec...
Embodiment 3
[0151] (1) Magnetic stirring device, add 100mL of absolute ethanol to a 250mL three-necked reaction flask, add 72.14g (0.3mol, 1eq) of chloropropylmethyldimethoxysilane and 0.45g of capping agent hexamethylsiloxane Alkanes, 77.8g of concentrated hydrochloric acid was slowly added dropwise to the reaction liquid, and the reaction was refluxed overnight, and the reaction was completed in about 16 hours. Most of the solvent was removed by rotary evaporation with ethanol and water, and dried in a vacuum oven at 80°C for about 15 hours to obtain a colorless transparent liquid chloropropyl polymethylsiloxane for future use.
[0152] (2) R 1 Preparation of potassium salt: magnetic stirring device, 100mL of absolute ethanol, 31.4g (0.2mol, 1eq) of 2,2,6,6-tetramethylpiperidinol were added to a 250mL three-neck reaction flask, and hydrogen was added to the reaction solution Potassium oxide 11.2g (0.2mol, 1eq), stirred at room temperature to a colorless and clear solution, the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com