Pyridine-ion-containing liquid, preparation method and application thereof
A technology of pyridine ions and liquids, applied in the field of chemical materials, can solve the problem of high melting point of ionic liquids, achieve the effect of improving complexation ability, suitable for industrial production, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] see figure 1 , figure 1 The flowchart showing the preparation method of the pyridine-containing ionic liquid according to the embodiment of the present invention includes the following steps:
[0025] Step S01, preparing alkylpyridine halides
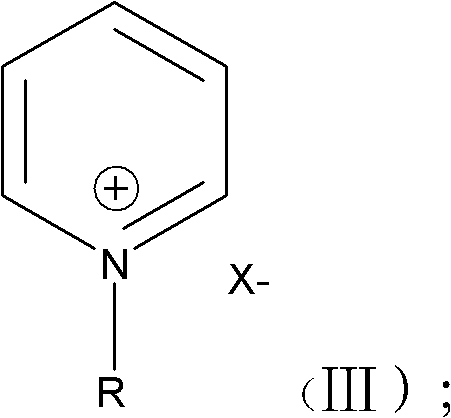
[0026] Under anhydrous and oxygen-free conditions, mix pyridine and haloalkane in a molar ratio of 1:1.05-1:1.2, stir and react at a temperature of 60-80°C for 48-72 hours, and wash to obtain the alkylpyridine represented by structural formula (III) Halides, wherein the alkyl halide is selected from one of methoxyethoxymethyl chloride, methoxyethoxymethyl bromide or methoxyethoxymethyl iodide, and the structural formula (III) is:
[0027]
[0028] Step S02, preparing an ionic liquid containing pyridine
[0029] The alkylpyridine halide represented by the structural formula (III) and the inorganic salt are dissolved in water, stirred and reacted for 8-24 hours to obtain a pyridine-containing ionic liquid, wherein the anion i...
Embodiment 1
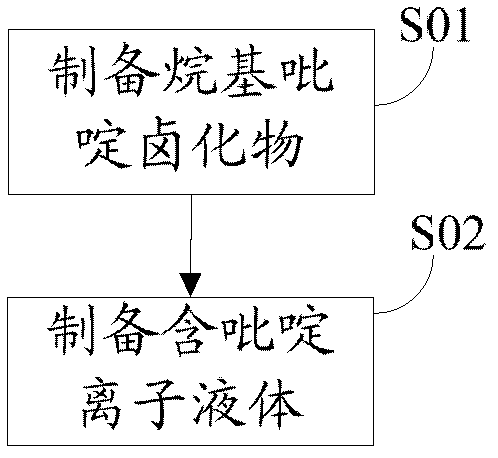
[0042] The embodiment of the present invention contains pyridine ionic liquid, which has the following structural formula:
[0043]
[0044] The preparation method of the pyridine-containing ionic liquid according to the embodiment of the present invention comprises the following steps:
[0045] Step 1, preparation of chlorinated 1-methoxyethoxymethylpyridinium chloride salt
[0046] in N 2 (or Ar 2 ) under the protection of atmosphere, pyridine (79g, 1mol) and methoxyethoxymethane chloride (136.4g, 1.1mol) were added to the reactor, the temperature was adjusted to 60°C, and the reaction was stirred for 48 hours; standing for cooling, Then the reaction product was washed three times with ethyl acetate, and dried under vacuum at 80° C. to obtain a pale yellow solid with a yield of 85%;
[0047] Step 2, preparation of 1-methoxyethoxymethylpyridine tetrafluoroborate
[0048] The 1-methoxyethoxymethylpyridine chloride (112.5g, 0.5mol) obtained in step 1, NaBF 4 (55g, 0.5mo...
Embodiment 2
[0051] The embodiment of the present invention contains pyridine ionic liquid, which has the following structural formula:
[0052]
[0053] The preparation method of the pyridine-containing ionic liquid according to the embodiment of the present invention comprises the following steps:
[0054] Step 1, preparation of 1-methoxyethoxymethylpyridine bromide
[0055] in N 2 (or Ar 2 ) under the protection of atmosphere, pyridine (79g, 1mol) and methoxyethoxymethyl bromide (184.8g, 1.1mol) were added to the reactor, the temperature was adjusted to 70°C, and the reaction was stirred for 60 hours; standing for cooling, and then The reaction product was washed three times with ethyl acetate, and dried under vacuum at 80°C to obtain a light yellow solid with a yield of 86%;
[0056] Step 2, preparation of 1-methoxyethoxymethylpyridine tetrafluoroborate
[0057] The brominated 1-methoxyethoxymethylpyridine (0.5mol), NaBF 4 (55g, 0.5mol), and 120mL deionized water were added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com