Flavonoids from nervilia fordii and preparation method and use thereof
A technology of flavonoids and uses, applied in the field of medicine, can solve the problems of rare antibacterial experimental research, retention, and few evaluations of antibacterial activity, and achieve good medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Take 5 kg of dry whole herb of Nervilia fordii (Hance) Schltr., add 10 times the amount of 60% ethanol to reflux and extract 3 times, each time for 2 hours, combine the extracts, and use a rotary evaporator at a temperature lower than 45 ° C. After recovering ethanol under reduced pressure, 600 g of extract was obtained; the extract was suspended in water to obtain an aqueous suspension of extract, and the aqueous suspension of extract was extracted three times with cyclohexane, and the cyclohexane layers were combined; Ethyl extracts the aqueous extract suspension after extracting the extract aqueous suspension with cyclohexane three times, and combines the ethyl acetate layer; then uses n-butanol to extract the extract aqueous suspension with ethyl acetate The extract water suspension was extracted three times, and the n-butanol layers were combined; the cyclohexane layer solvent cyclohexane, the ethyl acetate layer solvent ethyl acetate, and the After the butanol lay...
Embodiment 2
[0054] Take 10kg of dried Nervilia fordii (Hance) Schltr. whole herb, add 7 times the amount of 80% ethanol to reflux and extract twice, each time for 3 hours, combine the extracts, and use a rotary evaporator at a temperature lower than 45°C After recovering ethanol under reduced pressure, 1200 g of extract was obtained. Suspend the extract in water to obtain an aqueous suspension of extract, extract the aqueous suspension of extract three times with cyclohexane, combine the cyclohexane layers; then extract the aqueous extract with cyclohexane with ethyl acetate The aqueous extract suspension after the suspension was extracted three times, and the ethyl acetate layer was combined; then, the aqueous extract suspension was extracted three times with n-butanol after the aqueous extract suspension was extracted with ethyl acetate, and the normal ethyl acetate layer was combined. butanol layer; use a rotary evaporator to recover the cyclohexane layer solvent cyclohexane, the ethyl...
experiment example 1
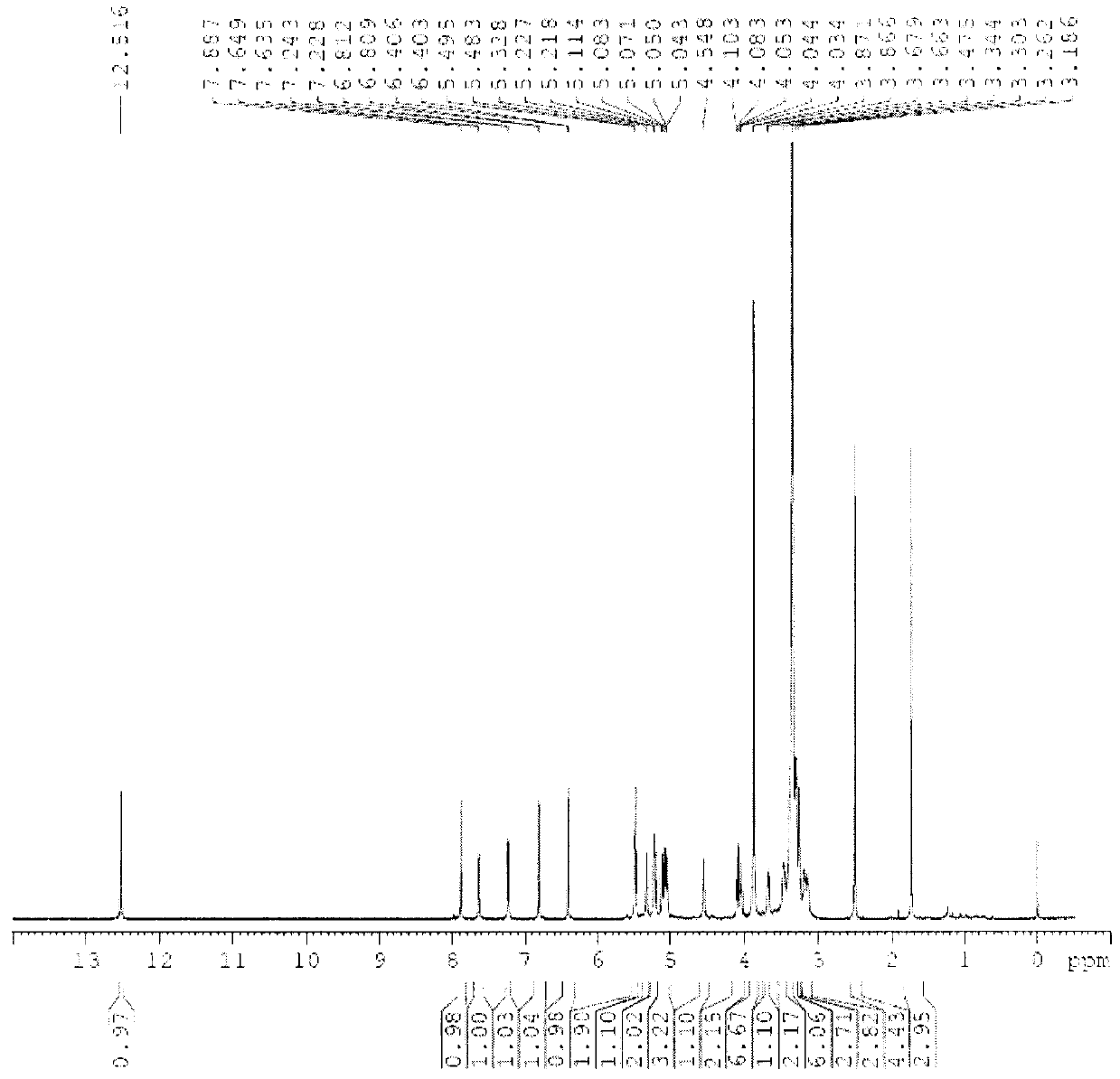
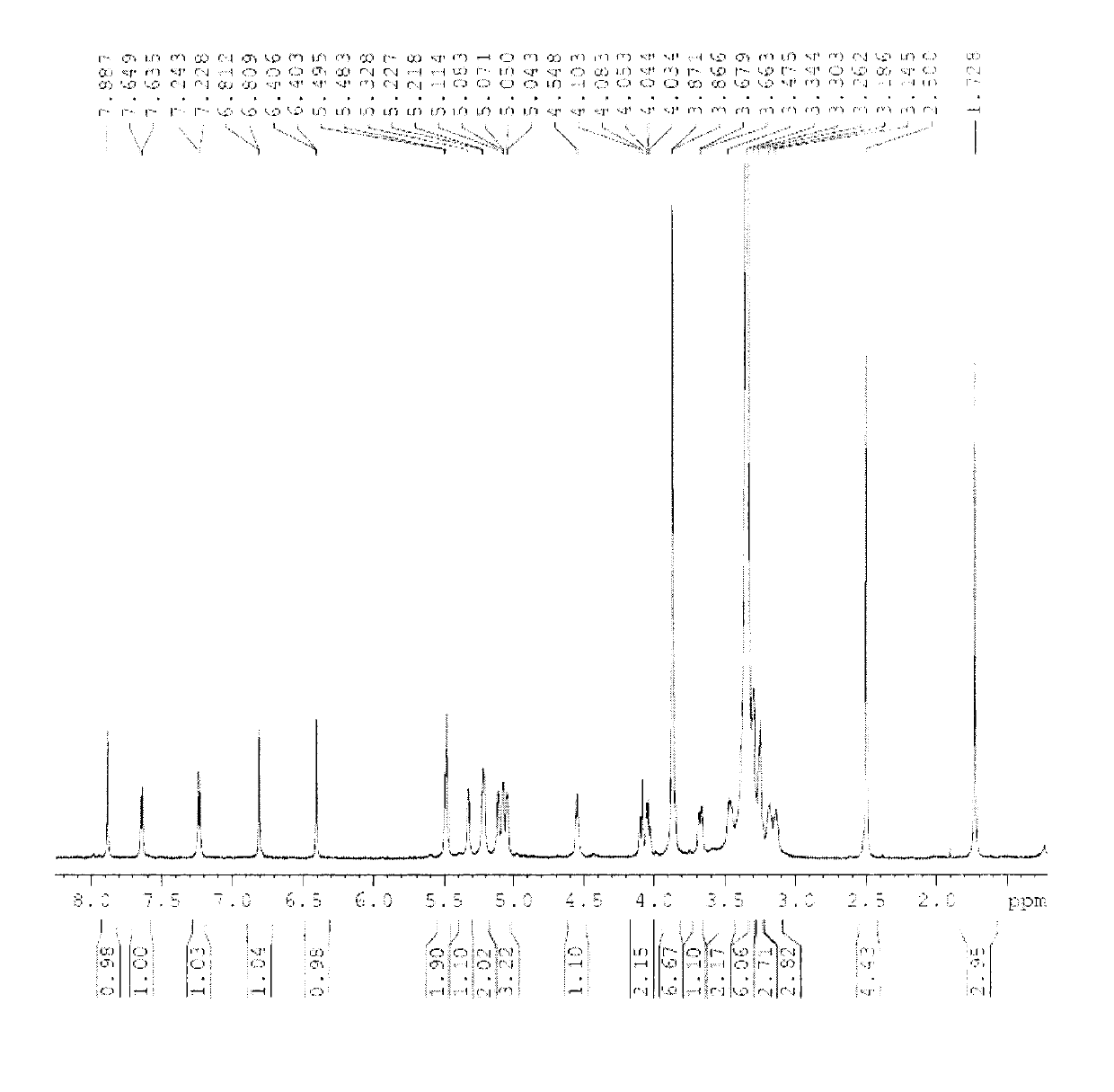
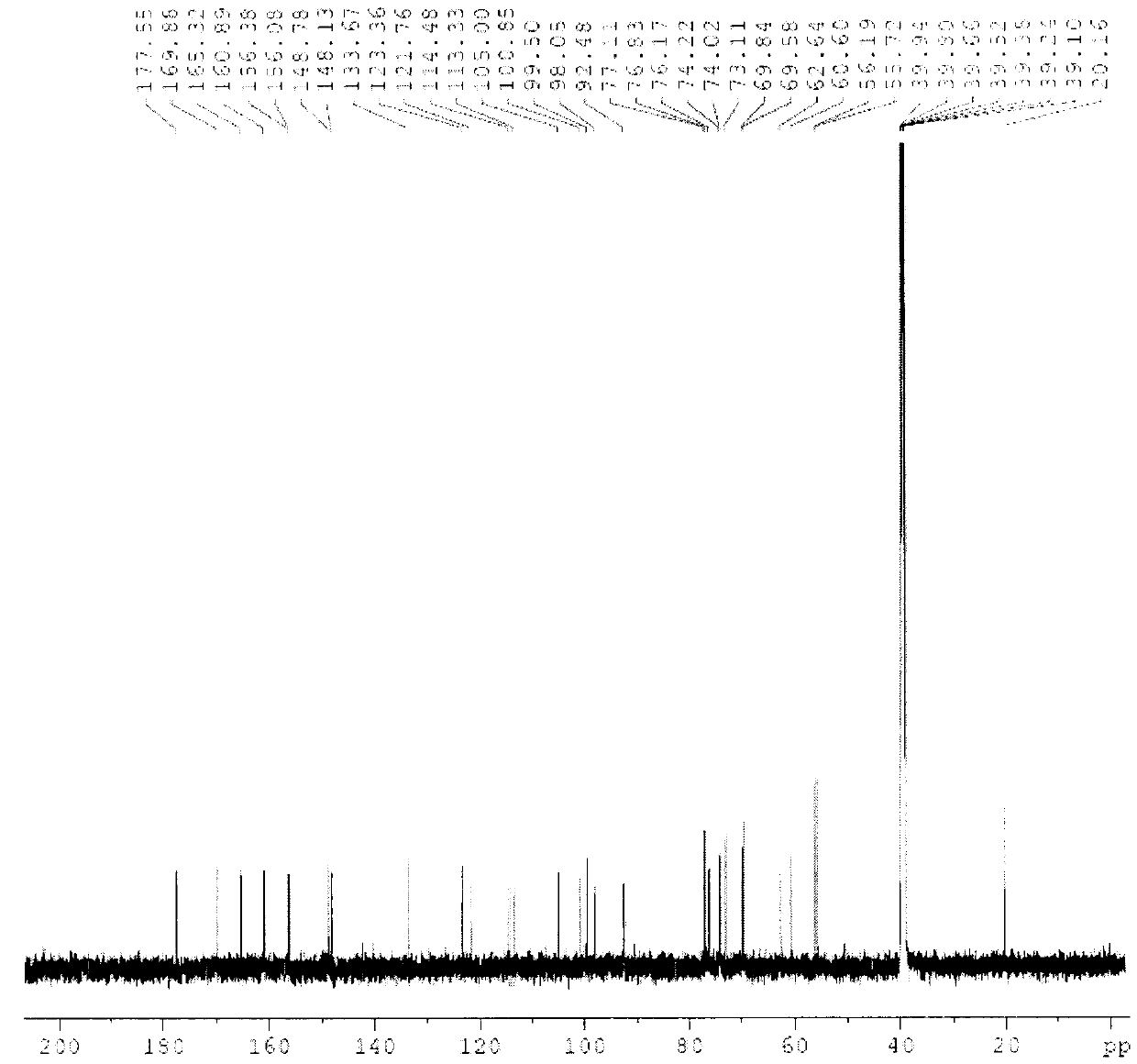
[0059] Compound 1, yellow powder, odorless, melting point: 154°C-156°C (methanol). High-resolution ESI-MS gives molecular ion peaks: [M+H + ]m / z 697.2689, the molecule is: C 31 h 36 o 18 , Molecular weight: 696.61. IR spectrum at 3393cm -1 There is a strong broad peak, suggesting that there is a hydroxyl group in the molecule, 1655cm -1 The strong absorption peaks at 1600 and 1499cm suggest that there is a carbonyl group in the structure -1 The absorption peaks suggest that there is a benzene ring in the structure. The chemical name of compound 1 is: (3,4,5-trihydroxy-6-(7-methoxy-5-hydroxy-2-(3-methoxy-4-(3,4,5-trihydroxy -6-Hydroxymethyl-tetrahydro-2H-pyran-2-acyloxy)phenyl)-4-oxo-4H-benzopyran-3-acyloxy)-tetrahydro-2H-pyran ) methyl acetate.
[0060] of the compound 1 H-NMR (600MHz, DMSO-d 6 ), δ12.52 (1H, s) is the characteristic signal of the 5-hydroxyl proton of flavone, δ6.81 (1H, d, J = 2.0Hz), 6.40 (1H, d, J = 2.0Hz) is the 5, 7 di Oxygen-substituted flavo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com