Spherical lithium-enriched anode material with gradient concentration and preparation method thereof
A lithium-rich positive electrode material with a gradual change in concentration technology, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of low compaction density, environmental pollution, and low tap density of ternary materials, and achieve excellent cycle life and thermal stability. The effects of stability, simple and easy-to-control preparation process, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
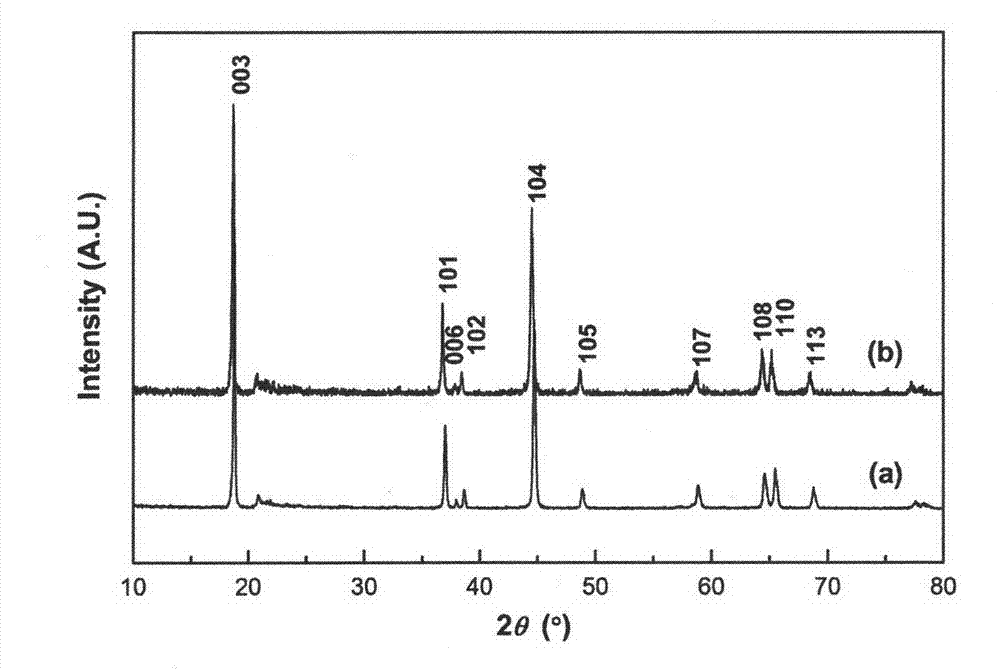
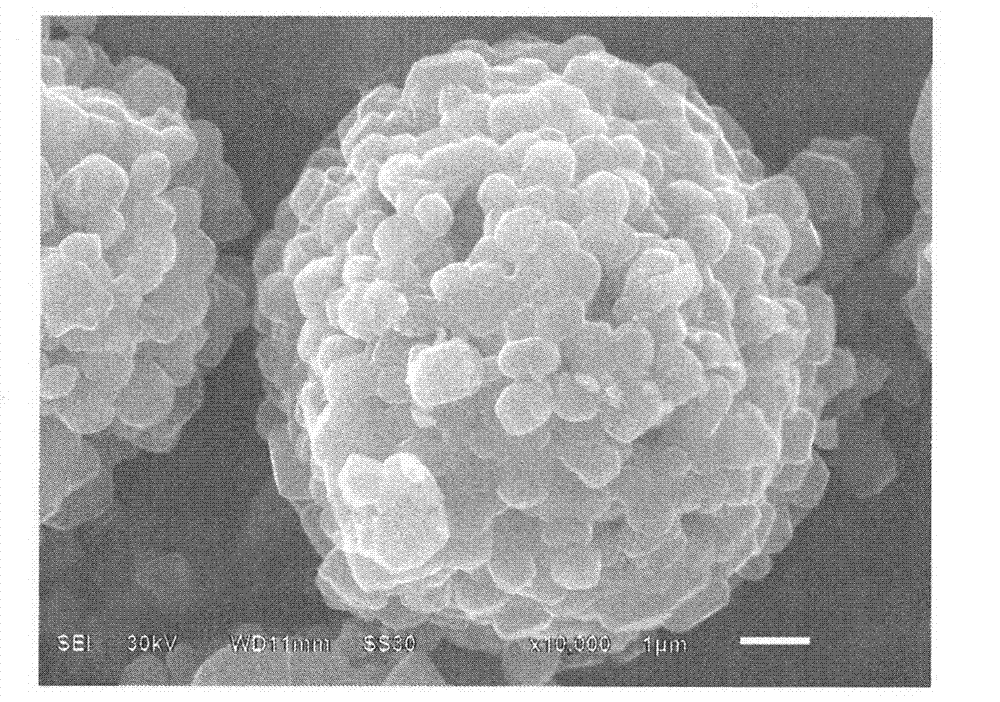
Image
Examples
Embodiment 1
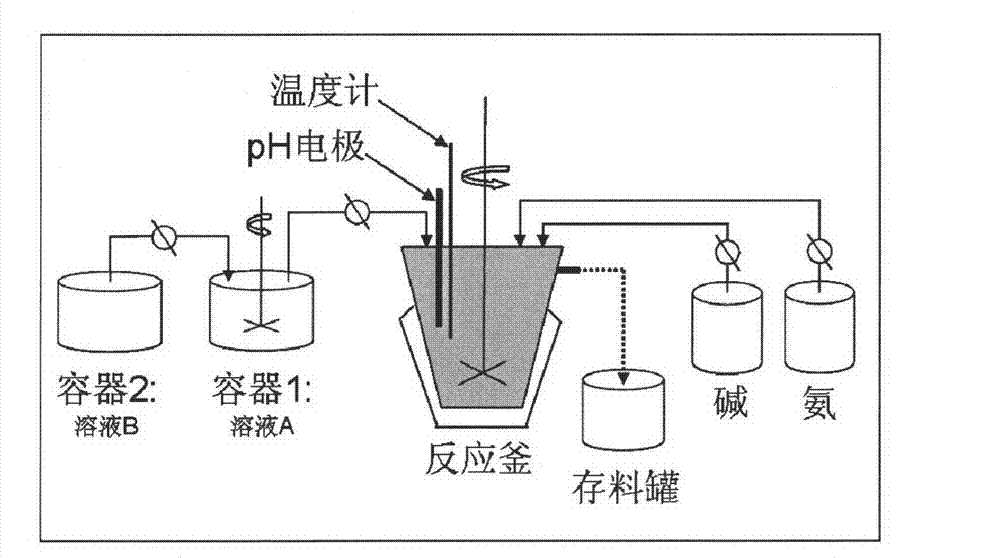
[0026] (1) nickel sulfate (NiSO 4 ·6H 2 O), manganese sulfate (MnSO 4 ·H 2 O), cobalt sulfate (NiSO 4 ·7H 2 O) mix by Ni: Mn: Co (molar ratio)=1: 1: 1 ratio, be dissolved in deionized water, be mixed with the mixed salt solution A that total metal ion concentration is 1.5mol / L; Take a certain amount of sulfuric acid Manganese (MnSO 4 ·H 2 O) be dissolved in deionized water, be mixed with the manganese salt solution B that total metal ion concentration is 1.5mol / L; Prepare the Na that concentration is 1.5mol / L respectively 2 CO 3 Alkaline solution and ammonia water with a concentration of 0.3mol / L.
[0027] (2) Add the manganese salt solution B (500mL) prepared in step (1) into the mixed salt solution A (500mL) under stirring through a constant flow pump, and at the same time, mix the mixed salt solution A and B The solution is added into the reaction kettle through a constant flow pump, Na 2 CO 3 Alkali solution and ammonia water are respectively fed into the reacti...
Embodiment 2
[0032] (1) nickel sulfate (NiSO 4 ·6H 2 O), manganese sulfate (MnSO 4 ·H 2 O) mix by Ni:Mn (molar ratio)=1:1 ratio, dissolve in deionized water, be mixed with the mixed salt solution A that the total metal ion concentration is 2.0mol / L; Take a certain amount of manganese sulfate (MnSO 4 ·H 2 O) be dissolved in deionized water, be mixed with total metal ion concentration and be the salt solution B of 2.0mol / L; Prepare the Na that concentration is 2.0mol / L respectively 2 CO 3 Alkaline solution and ammonia water with a concentration of 0.4mol / L.
[0033] (2) Add the manganese salt solution B (400mL) prepared in step (1) into the mixed salt solution A (600mL) under stirring through a constant flow pump, and at the same time, mix the mixed salt solution A and B The solution is added into the reaction kettle through a constant flow pump, Na 2 CO 3 Alkaline solution and ammonia water are respectively fed into the reaction kettle in parallel through a constant flow pump, the s...
Embodiment 3
[0037] (1) nickel sulfate (NiSO 4 ·6H 2 O), manganese sulfate (MnSO 4 ·H 2 O), cobalt sulfate (NiSO 4 ·7H 2 O) mix by Ni: Mn: Co (molar ratio)=4: 4: 2 ratio, be dissolved in deionized water, be mixed with the mixed salt solution A that total metal ion concentration is 2.0mol / L; Take a certain amount of sulfuric acid Manganese (MnSO 4 ·H 2 O) be dissolved in deionized water and be prepared into a salt solution B with a total metal ion concentration of 2.0mol / L; prepare a NaOH alkali solution with a concentration of 4.0mol / L and ammoniacal liquor with a concentration of 4.0mol / L respectively.
[0038] (2) Add the manganese salt solution B (300mL) prepared in step (1) into the mixed salt solution A (700mL) under stirring through a constant flow pump, at the same time, mix the mixed salt solution A and B The solution is added to the reactor through a constant flow pump, NaOH alkali solution and ammonia water are respectively fed into the reactor in parallel through a consta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com