Method for preparing C20 position dehydroxylation dammarane type rare ginsenoside and aglycone thereof
A dehydration reaction and compound technology, applied in the field of natural medicine, can solve the problems of difficult separation, poor purity, low yield, etc., and achieve the effect of simple reaction steps and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
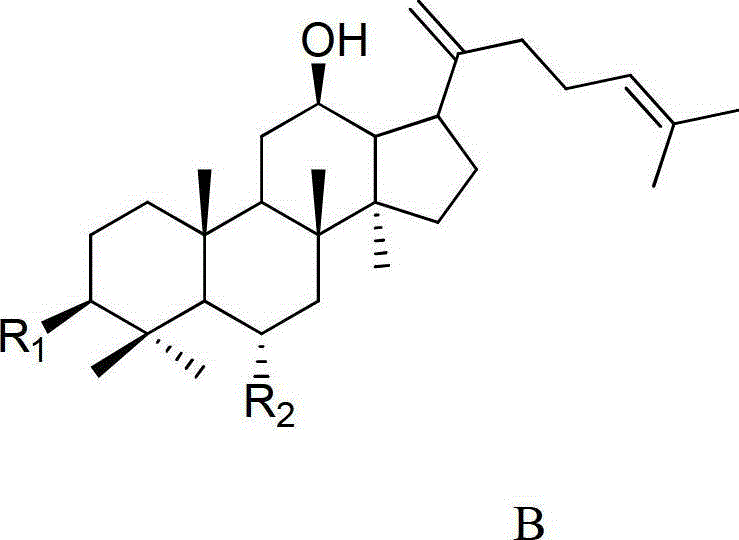
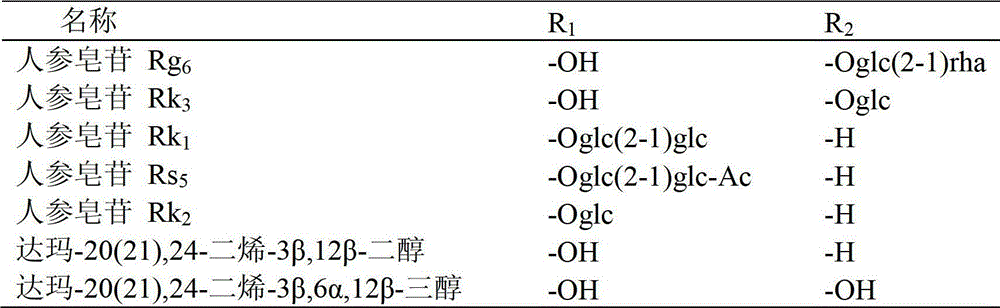
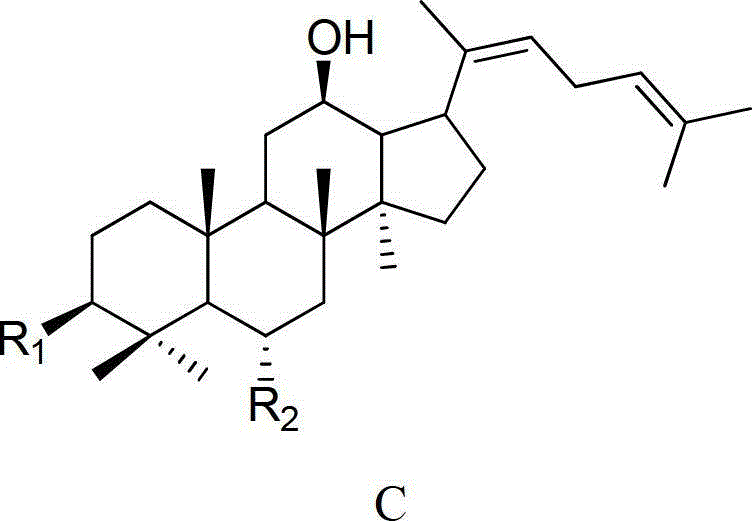
[0042] 20mg ginsenoside Rh 1Respectively dissolve 40ml of 0.05% formic acid in methanol: water (V:V4:6) mixed solution, and heat at 120°C for 4 hours under the pressure of 0.12MPa. After the reaction liquid is cooled, select Agilent Zorbax SB-C18 semi-preparative column (250mm × 9.4mm I.D., 5 μ m), use mobile phase as the aqueous solution containing 50% acetonitrile for isocratic elution, and wait for the raw material Rh 1 After the peak, collect the two main chromatographic peaks (the former is ginsenoside Rk 3 , the latter being ginsenoside Rh 4 ), concentrated and freeze-dried to get a white powder, the weights are Rk 3 1.70mg (8.75% yield), Rh 4 3.97 mg (yield 20.44%), the purity determined by HPLC method was 98.9% and 92.7%, respectively. After MS, 13 C-NMR measurement (relevant data is attached), and verified with relevant literature data, confirmed that the white powder is ginsenoside Rk 3 and Rh 4 .
[0043] Ginsenoside Rk 3 Identification of:
[0044] White ...
Embodiment 2
[0050] 50mg ginsenoside Rg 3 Dissolve in 75ml of 0.02% formic acid ethanol: water (V:V5:5) mixed solution, and heat at 120°C for 6 hours under the pressure of 0.12MPa. After the reaction solution is cooled, select Agilent Zorbax SB-C18 semi-preparative column (250mm × 9.4mm I.D., 5 μm), use the mobile phase as the aqueous solution containing 65% acetonitrile for isocratic elution, and wait for the raw material Rg 3 After the peak, collect the two main chromatographic peaks (the former is ginsenoside Rk 1 , the latter being ginsenoside Rg 5 ), concentrated and freeze-dried to get a white powder, the weights are Rk 1 3.96mg (8.11% yield), Rg 5 8.01 mg (yield 16.40%), the purity determined by HPLC method was 94.7% and 98.5%, respectively. After MS, 13 C-NMR measurement (relevant data is attached), and verified with relevant literature data, confirmed that the white powder is ginsenoside Rk 1 and Rg 5 .
[0051] Ginsenoside Rk 1 Identification of:
[0052] White powder, ...
Embodiment 3
[0058] 100mg ginsenoside Rh 2Dissolve in 150ml of 0.01% formic acid methanol:water (V:V6:4) mixed solution, and heat at 120°C for 4 hours under 0.15MPa pressure. After the reaction liquid is cooled, select Agilent Zorbax SB-C18 semi-preparative column (250mm × 9.4mm I.D., 5 μ m), use mobile phase as the aqueous solution containing 80% acetonitrile for isocratic elution, and wait for the raw material Rh 2 After the peak, collect the two main chromatographic peaks (the former is ginsenoside Rk 2 , the latter being ginsenoside Rh 3 ), concentrated and freeze-dried to get a white powder, the weights are Rk 2 9.37mg (9.63% yield), Rh 3 14.01 mg (yield 14.39%), the purity determined by HPLC method was 96.8% and 98.7%, respectively. After MS, 13 C-NMR measurement (relevant data is attached), and verified with relevant literature data, confirmed that the white powder is ginsenoside Rk 2 and Rh 3 .
[0059] Ginsenoside Rk 2 Identification of:
[0060] White powder, easily sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com