Application of hexahydric cyclic carbonate in biodegradable materials
A biodegradable material, cyclic carbonate technology, applied in the field of medical polymer materials, can solve problems such as side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of 5,5'-oxydimethylenebis(5-ethyl-1,3-dioxan-2-one)
[0029] The reaction equation is
[0030]
[0031] 22.5 g (0.09 mol) of bis(trimethylolpropane), 57.0 g (0.53 mol) of ethyl chloroformate and 600 ml of tetrahydrofuran were mixed in a 1000 ml three-necked flask. The three-necked flask was placed in an ice-salt bath, and stirring was started to stabilize the temperature at -10°C. 56.0g (0.55mol) triethylamine and 100ml tetrahydrofuran were placed in a constant pressure titration funnel, and slowly added dropwise to the three-necked flask at -10°C, ensuring that the temperature did not exceed 0°C during the dropwise addition. After the dropwise addition, the temperature was naturally raised to room temperature, and the reaction was continued for 12-24 h. After completion of the reaction, suction filtration with a 1000ml suction filter bottle to generate triethylamine salt by filtration, the filtrate is concentrated by rotary evaporation and th...
Embodiment 2
[0032] Example 2 Preparation of 5,5'-oxydimethylene bis(5-ethyltrimethylene carbonate) homopolymeric cross-linked product
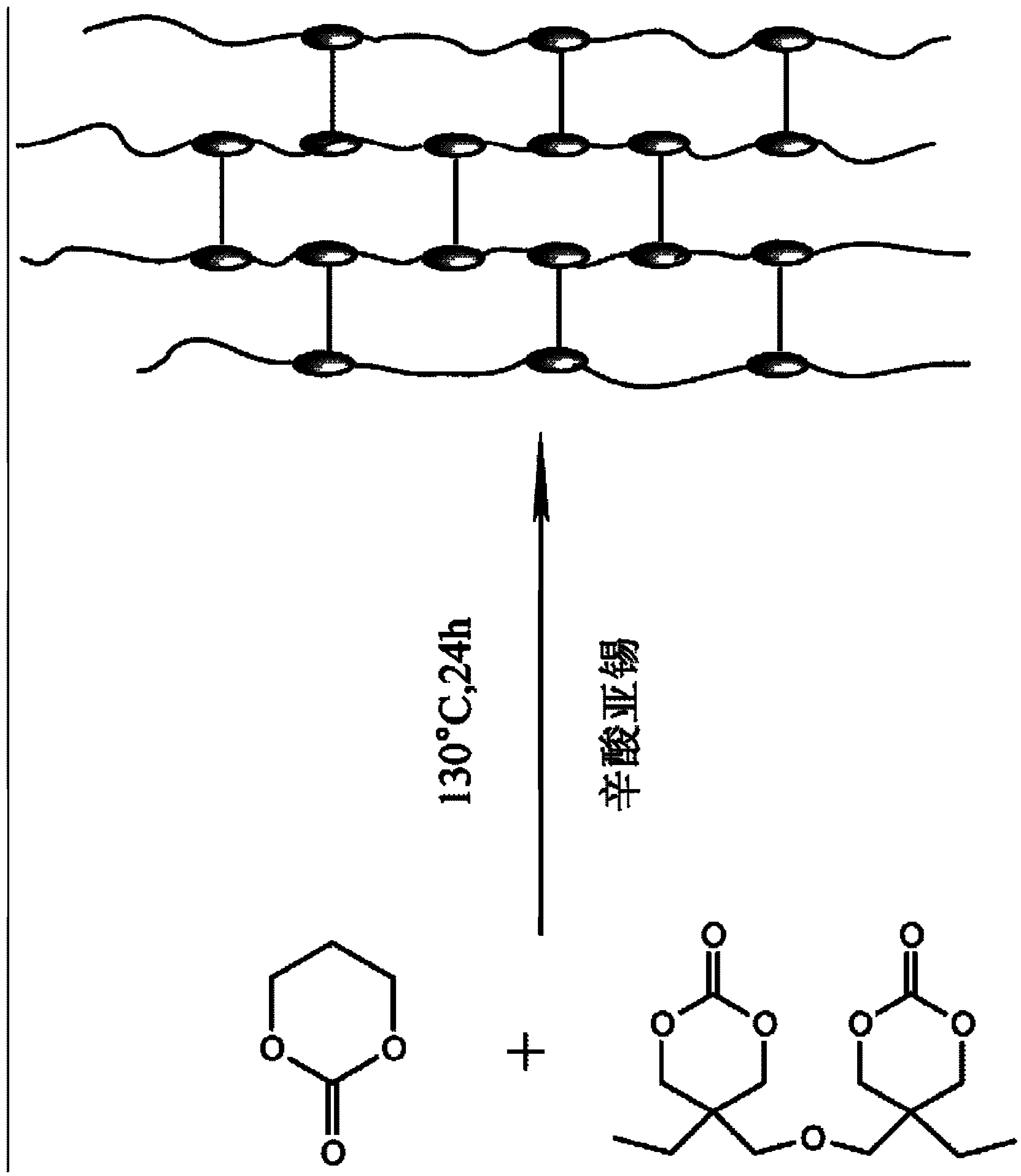
[0033] Under anhydrous and oxygen-free conditions, 0.1 mol of 5,5'-oxydimethylene bis(5-ethyltrimethylene carbonate) was added to the polymerization tube, and stannous octoate was added after decompression and vacuuming for 3 times. (The molar ratio of monomer to stannous octoate is 5000:1), evacuated under reduced pressure for 3 times (vacuum degree<15Pa), and sealed the tube under vacuum condition. Bulk polymerization was carried out at 130° C. for 24 h to obtain a homopolymerized cross-linking product of the cross-linking agent.
Embodiment 3
[0034] Example 3 Cross-linking polymerization of 5,5'-oxydimethylene bis(5-ethyl-1,3-dioxan-2-one) and trimethylene carbonate
[0035] For the reaction equation see figure 2 . Under anhydrous and anaerobic conditions, 0.2mol trimethylene carbonate and 5×10 -5 mol5,5'-oxydimethylene bis(5-ethyl-1,3-dioxane-2-one) was added into the polymerization tube, and stannous octoate ( The ratio of the total number of moles of trimethylene carbonate and crosslinking agent to the moles of stannous octoate is 5000:1), evacuated under reduced pressure for 3 times (vacuum degree<15Pa), and sealed under vacuum conditions. Polymerization at 130 °C for 24 h to obtain cross-linking of 5,5'-oxydimethylenebis(5-ethyl-1,3-dioxan-2-one) with trimethylene carbonate polymer.
[0036] By adjusting the amount of 5,5'-oxydimethylenebis(5-ethyl-1,3-dioxan-2-one) used, crosslinked polymers with different degrees of crosslinking can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com