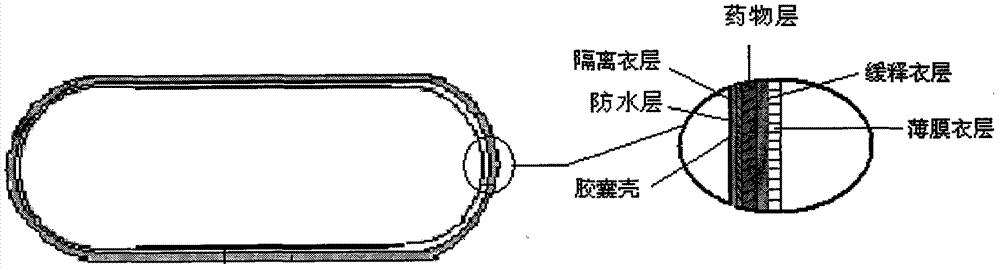
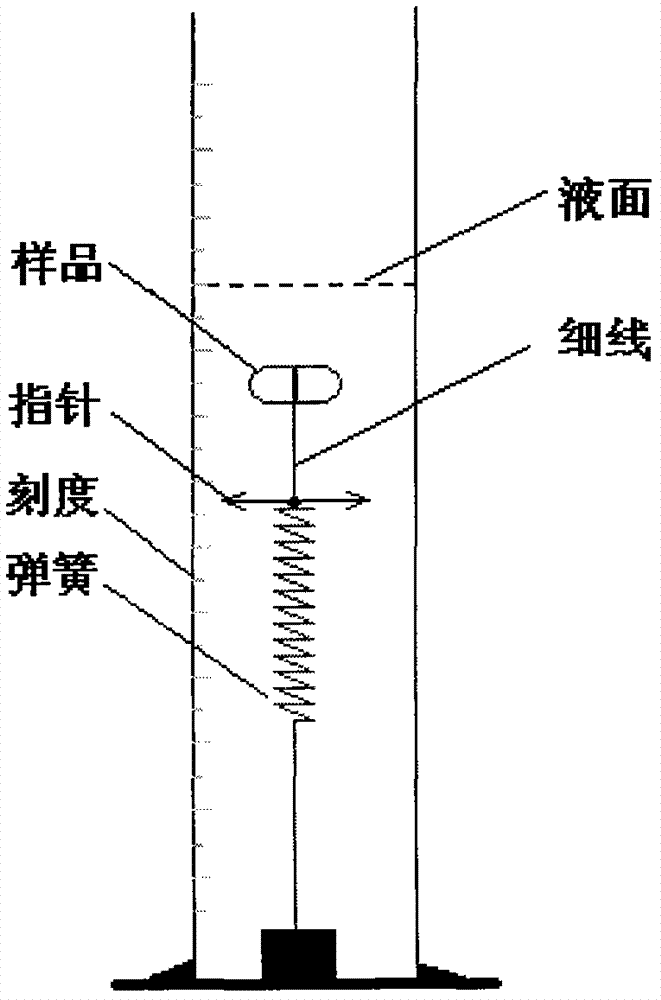
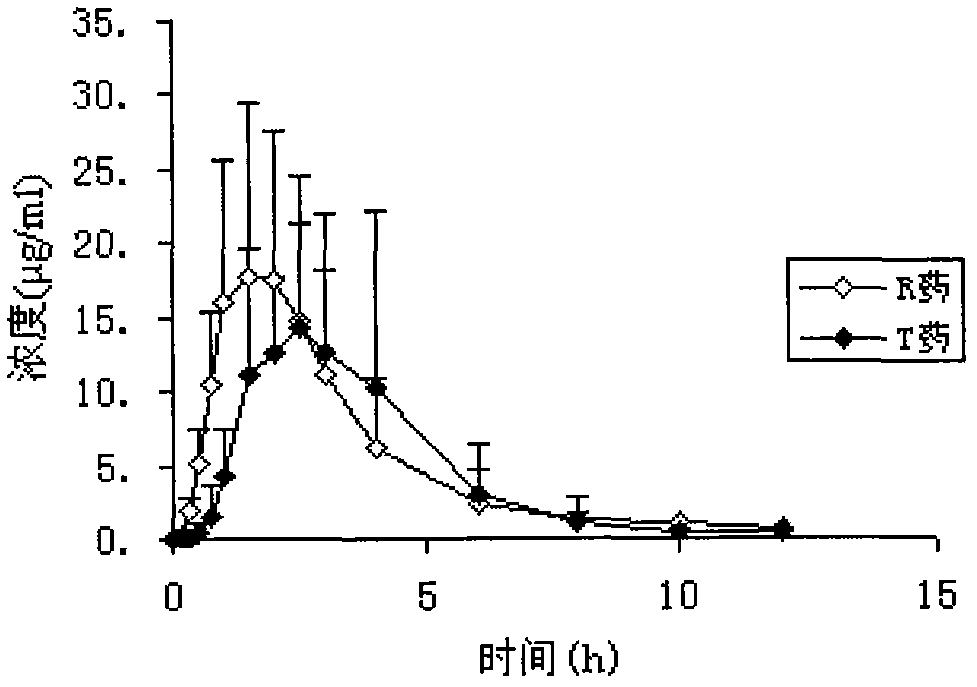
Faropenem Sodium Gastric Floating Sustained Release Preparation and Preparation Method
A technology of faropenem sodium and sustained-release preparations, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, antibacterial drugs, etc., can solve the problems of destroying normal intestinal flora, intestinal adverse reactions, etc. Part entry, weight reduction, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the investigation of drug-containing layer composition
[0054] 1. Prescription (1000 capsules)
[0055] Waterproof air bag prescription:
[0056] Capsule No. 2
1000
Ethylcellulose N-100
3.0g
5.0g
95% ethanol solution
100ml
[0057] Isolation gown layer prescription:
[0058] Hypromellose E5
50g
100g
100g
95% ethanol solution
400ml
water
600ml
[0059] Drug-containing layer prescription:
123.5g*
polyethylene glycol 4000
48g
Absolute ethanol
1600ml
[0061] *: Calculated as 100g by faropenem
[0062] Second, the preparation process:
[0063] 1. Preparation of waterproof air bag: put the No. 2 capsule into the coating pan, spray the prepared waterproof coating solution (made by dispersing stearic acid and ethyl cellulose in 95% ethanol) at a tablet bed tempe...
Embodiment 2
[0071] Embodiment 2: the investigation of drug-containing layer composition
[0072] 1. Prescription (1000 capsules)
[0073] Waterproof air bag prescription: with embodiment 1
[0074] Isolation gown layer prescription: with embodiment 1
[0075] Drug-containing layer prescription:
123.5g
talcum powder
3g
Povidone K30
48g
Absolute ethanol
1600ml
[0077] Second, the preparation process:
[0078] 1. Preparation of waterproof airbag: same as Example 1;
[0079] 2. Prepare the isolation gown layer: the same as in Example 1;
[0080] 3. Preparation of the drug-containing layer: Dissolve the raw material drug of faropenem sodium in absolute ethanol, add povidone K30 to dissolve, add talcum powder and mix well to prepare the coating liquid of the drug-containing layer. Under the condition of avoiding light, use a conventional film Coating method The drug-containing layer is wrapped outside the waterproof a...
Embodiment 3
[0084] Embodiment 3: the investigation of drug-containing layer composition
[0085] 1. Prescription (1000 capsules)
[0086] Waterproof air bag prescription: with embodiment 1
[0087] Isolation gown layer prescription: with embodiment 1
[0088] Drug-containing layer prescription:
[0089] Faropenem Sodium
123.5g
Hypromellose-SL
48g
Absolute ethanol
1600ml
[0090] Second, the preparation process:
[0091] 1. Preparation of waterproof airbag: same as Example 1;
[0092] 2. Prepare the isolation gown layer: the same as in Example 1;
[0093] 3. Preparation of the drug-containing layer: Dissolve the raw drug of faropenem sodium in absolute ethanol, add hydroxypropyl cellulose-SL to dissolve, add talcum powder and mix to prepare the coating solution of the drug-containing layer. The film coating method wraps the drug-containing layer outside the waterproof air bag wrapped with the isolation coat layer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com