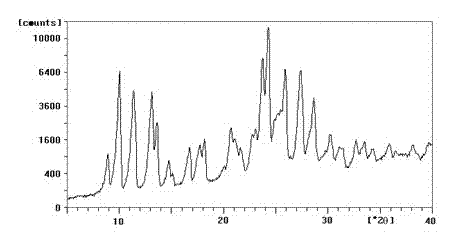
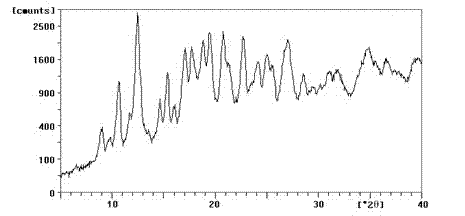
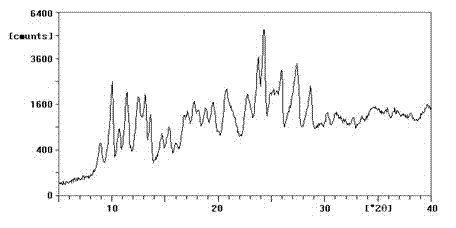
Enrofloxacin/sulfobutylether-beta-cyclodextrin inclusion compound, preparation method and medicinal preparation thereof
A technology of cyclodextrin inclusion complex and sulfobutyl ether, which is applied in the preparation method and pharmaceutical preparation thereof, in the field of enrofloxacin/sulfobutyl ether-β-cyclodextrin inclusion complex, and can solve the problem of poor solubility in water and other problems, to achieve the effect of improved stability, good curative effect and good oral palatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Weigh 6 grams of sulfobutyl ether-β-cyclodextrin (substitution degree 7), 1 gram of enrofloxacin, and dissolve them in 75 ml of water and 25 ml of ethanol respectively, and mix the two solutions after dissolving , heated and stirred at 55°C for 1 hour, cooled to room temperature, and filtered to obtain a solution, which was then spray-dried to obtain enrofloxacin / sulfobutyl ether-β-cyclodextrin inclusion compound.
Embodiment 2
[0048] Example 2: Weigh 3 grams of sulfobutyl ether-β-cyclodextrin (degree of substitution: 4), 1 gram of enrofloxacin, and dissolve them in 65 ml of water and 35 ml of methanol respectively, and mix the two solutions after dissolving , heated and stirred at 45°C for 36 hours, cooled to room temperature, filtered to obtain a solution, and the obtained solution was freeze-dried, which was enrofloxacin / sulfobutyl ether-β-cyclodextrin inclusion compound.
Embodiment 3
[0049] Example 3: Weigh 1 gram of sulfobutyl ether-β-cyclodextrin (substitution degree 7), 1 gram of enrofloxacin, and dissolve them in 25 ml of water and 75 ml of propanol respectively, and mix the two The solution was heated and stirred at 65°C for 2 hours, cooled to room temperature, filtered to obtain the solution, and the obtained solution was freeze-dried, which was enrofloxacin / sulfobutyl ether-β-cyclodextrin inclusion compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Lumen diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com