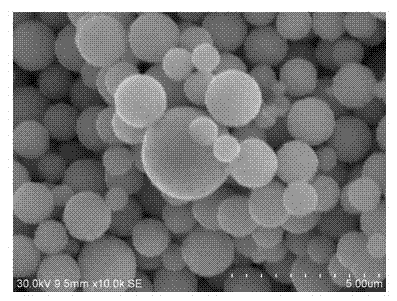
Silver powder for printing size for solar cell electrodes and preparation process thereof
A technology of solar cells and silver powder, applied in the field of solar cells, can solve the problems of difficult effective transmission of current and low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A. Prepare respectively the silver nitrate solution that is 0.5mol / L and the ascorbic acid solution that concentration is 0.5mol / L with deionized water;
[0025] B. Get 1% polyvinylpyrrolidone of the silver nitrate quality, add in the silver nitrate solution obtained in step A, and disperse for 5 minutes with a rotating speed of 450r / min under ultrasonic conditions, then adjust the pH of the mixed solution with dilute nitric acid to be 2, and obtain Silver precursor solution;
[0026] C. Get 1% polyvinylpyrrolidone of reducing agent quality, add in the reducing agent solution obtained in step A, obtain reducing mixed solution;
[0027] D. According to the molar ratio of reducing agent:silver nitrate=1:1, the silver precursor solution obtained in step B is gradually added dropwise to the reduction mixed solution obtained in step C for reaction, then left to stand, and then the liquid and solid are separated, and then the The solid was washed three times with deioniz...
Embodiment 2
[0029] A. Prepare respectively the silver nitrate solution that concentration is 1.5mol / L and the glucose solution that concentration is 0.6mol / L with deionized water;
[0030] B. Get 2.5% Tween of the silver nitrate mass, add it in the silver nitrate solution obtained in step A, and disperse for 5 minutes with a rotating speed of 500r / min under ultrasonic conditions, then adjust the pH of the mixed solution with dilute nitric acid to be 4 to obtain silver nitrate Precursor solution;
[0031] C. Take 2.5% Tween of reducing agent quality, add in the reducing agent solution obtained in step A, obtain reducing mixed solution;
[0032] D. According to the molar ratio of reducing agent:silver nitrate=2:1, the silver precursor solution obtained in step B is gradually added dropwise to the reduction mixed solution obtained in step C for reaction, then left to stand, and then the liquid and solid are separated, and then The solid matter was washed 3 times with deionized water and...
Embodiment 3
[0034] A. Prepare the silver nitrate solution that is 2mol / L and the triethanolamine solution that concentration is 0.7mol / L with deionized water respectively;
[0035] B. Get the polyethylene glycol of 5% of silver nitrate quality, add in the silver nitrate solution gained in step A, and under the condition of ultrasonic wave with rotating speed be that 600r / min carry out disperse for 5 minutes, then adjust the pH of mixed solution with dilute nitric acid to be 6, Obtain silver precursor solution;
[0036] C. Get 5% polyethylene glycol of reducing agent quality, add in the reducing agent solution obtained in step A, obtain reducing mixed solution;
[0037] D. According to the molar ratio of reducing agent: silver nitrate = 4: 1, the silver precursor solution obtained in step B is gradually added dropwise to the reduction mixed solution obtained in step C for reaction, then left to stand, and then the liquid and solid are separated, and then the The solid matter was washe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com