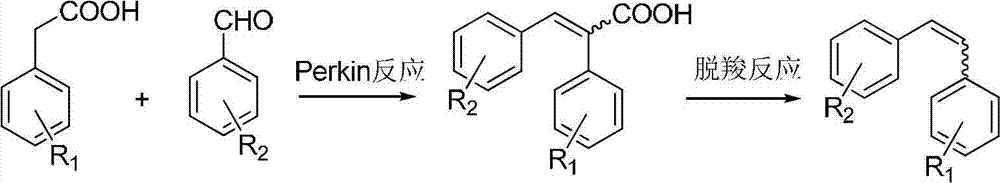
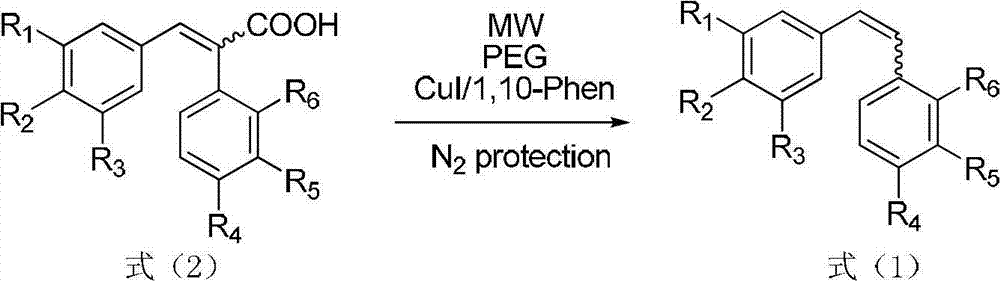
Method for preparing diphenyl ethylene compound through decarboxylic reaction
A technology of stilbenes and decarboxylation reaction, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of aminohydroxyl compounds. It can solve the problems of narrow substrate range, increased cost, and unrecoverable ligands, etc., and achieve saving Cost, convenient separation and purification, and wide range of substrate applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: (Z)-3,4,4', the preparation of 5-tetramethoxy-3'-aminostilbene
[0025] (E)-3-(3,4,5-trimethoxyphenyl)-2-(3'-amino-4'-methoxyphenyl)acrylic acid 1.795g (5mmol), cuprous iodide 0.0952 g (0.5mmol), o-phenanthroline 0.0991g (0.5mmol), PEG-40020mL were added to the reaction flask, and nitrogen protection was applied, and the reaction device was placed in a microwave synthesizer, and the power was set to 800W. The reaction was 6 minutes, and each reaction was 2 minutes. , intermittent 5min. After the reaction was completed, cool slightly, add 20mL of ice water and 20mL of ethyl acetate, filter with suction to obtain a brick red solid, rinse with distilled water 3 times, dry in the air, and recover. The filtrate was allowed to stand, and the ethyl acetate layer was separated, and the aqueous layer was extracted twice with ethyl acetate, 20 mL / time, the ethyl acetate layers were combined, dried with anhydrous magnesium sulfate, suction filtered, and concentrated...
Embodiment 2
[0027] Embodiment 2: (Z)-3,4,4', the preparation of 5-tetramethoxy-3'-aminostilbene
[0028] (E)-3-(3,4,5-trimethoxyphenyl)-2-(3'-amino-4'-methoxyphenyl)acrylic acid 1.795g (5mmol), recovered in Example 1 to obtain Cuprous iodide and o-phenanthroline complex 0.359g (20wt%), PEG-400 20mL is added in the reaction flask, feeds nitrogen protection, puts the reaction device into a microwave synthesizer, sets the power to 800W, and reacts for 8min. Each reaction is 2 minutes, and the interval is 5 minutes. Cool slightly after the reaction is complete, add 20mL ice water and 20mL ethyl acetate, filter with suction to obtain a brick red solid, rinse with distilled water 3 times, dry, recover, the filtrate is allowed to stand and the ethyl acetate layer is separated, and the water layer is then washed with acetic acid Ethyl extraction was performed twice, 20 mL each time, the ethyl acetate layers were combined, dried over anhydrous magnesium sulfate, suction filtered and concentrated ...
Embodiment 3
[0029] Example 3: Preparation of (E)-3,4,4',5-tetramethoxy-3'-aminostilbene
[0030] (Z)-3-(3,4,5-trimethoxyphenyl)-2-(3'-amino-4'-methoxyphenyl)acrylic acid 1.795g (5mmol), cuprous iodide 0.0952 g (0.5mmol), o-phenanthroline 0.0991g (0.5mmol), and PEG-40050mL were added to the reaction flask, and nitrogen protection was introduced, and the reaction device was placed in a microwave synthesizer, and the power was set at 800W, and the reaction was carried out for 6 minutes. 2min, intermittent 5min. Cool slightly after the reaction is complete, add 25ml of ice water and 25mL of ethyl acetate, filter with suction to obtain a brick red solid, rinse with distilled water 3 times, dry in the air, recover, let the filtrate stand still and separate the ethyl acetate layer, the water layer is then washed with acetic acid Ethyl extraction was carried out twice, 25 mL each time, the ethyl acetate layers were combined, dried over anhydrous magnesium sulfate, suction filtered and concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com