Schiff base, as well as preparation and applications of Schiff base as pickling inhibitor for steel products
A Schiff base and steel technology, applied in the preparation of imino compounds, organic chemistry, etc., can solve the problems of the urgency to find new corrosion inhibitors, the toxic effects of the environment and life systems, etc. Simple method and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
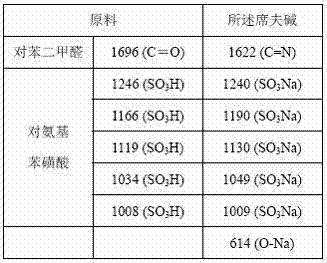
[0015] 1) Preparation of Schiff base. Dissolve 0.35g (2mmol) p-aminobenzenesulfonic acid and 0.08g (2mmol) NaOH solid in 15mL of anhydrous methanol to obtain solution A, and dissolve 0.134g (1mmol) terephthalaldehyde in 10mL of anhydrous methanol to obtain solution B Mix the two solutions of A and B in a round-bottomed flask, stir and reflux in a water bath at 40°C for 5 hours; then distill off half of the solvent from the mixture in the flask under reduced pressure, and obtain a light yellow powdery crude product after filtration and separation. The product was recrystallized in absolute ethanol to obtain the final product. Products and raw materials were characterized by potassium bromide tablet infrared spectroscopy, and the results are listed in the following table (cm -1 ):
[0016]
[0017] The data in the table shows that the carbonyl group (C=O) of terephthalaldehyde is transformed into the imino group (C=N) in the Schiff base, and the sulfonic acid group (SO 3 ...
Embodiment 2
[0020] 1) Preparation of Schiff base. Dissolve 0.38g (2.2mmol) of p-aminobenzenesulfonic acid and 0.088g (2.2mmol) of NaOH solid in 15mL of anhydrous methanol to obtain solution A, and dissolve 0.134g (1mmol) of terephthalaldehyde in 10mL of anhydrous methanol to obtain Solution B: Mix the two solutions of A and B in a round bottom flask, stir and reflux in a water bath at 60°C for 2 hours; then distill the mixture in the flask under reduced pressure to remove half of the solvent, and obtain a light yellow powder after filtration and separation The crude product was recrystallized in anhydrous methanol to obtain the final product Schiff base. The product was characterized by potassium bromide tablet infrared spectroscopy, and the obtained result was the same as that in Example 1, which proved that the Schiff base of the present invention was successfully synthesized.
[0021] 2) Corrosion inhibition performance testing. The corrosion inhibition performance detection of the S...
Embodiment 3
[0023] 1) Preparation of Schiff base. Dissolve 0.365g (2.1mmol) of p-aminobenzenesulfonic acid and 0.084g (2.1mmol) of NaOH solid in 15mL of absolute ethanol to obtain solution A, and dissolve 0.134g (1mmol) of terephthalaldehyde in 10mL of absolute ethanol to obtain Solution B: Mix the two solutions in a round bottom flask, stir and reflux in a water bath at 60°C for 5 hours; then distill the mixture in the flask under reduced pressure to remove half of the solvent, and obtain a light yellow powdery crude product after filtration and separation , recrystallized in absolute ethanol to obtain the final product Schiff base. The product was characterized by potassium bromide tablet infrared spectroscopy, and the obtained result was the same as that in Example 1, which proved that the Schiff base of the present invention was successfully synthesized.
[0024] 2) Corrosion inhibition performance testing. The addition amount of described Schiff's base is 0.049g, and other detectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com