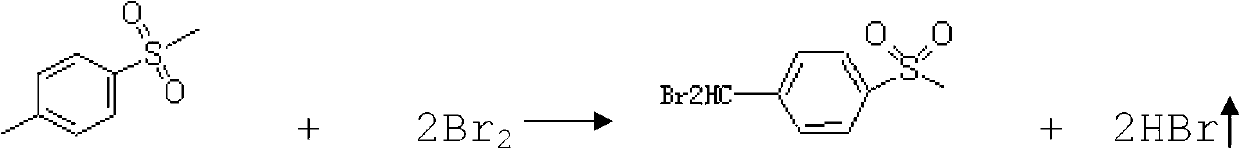
Method for synthesizing methyl sulfone base dibromo toluene
A technology of methylsulfonyl dibromotoluene and methylsulfonyl toluene, which is applied in the field of organic synthesis, can solve the problems that the production volume of methylsulfonyl dibromotoluene cannot meet the market demand, etc., and achieves easy industrial production, mild reaction conditions, and high product quality. high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under the temperature condition of 150 DEG C according to the molar ratio of 1:5 to p-thiamphenicol toluene and bromine, under the action of catalyst copper, heat preservation reaction was carried out for 8 hours to obtain liquid crude product p-thiamphenicol dibromotoluene, the catalyst The addition amount is 1wt% of p-thiamphenicol toluene.
[0026] Separating and removing the hydrobromic acid and unreacted bromine produced in the reaction from the liquid crude product p-thiamphenyl dibromide to obtain granular solid crude product p-thiamphenyl dibromide.
[0027] The solid crude product p-thiamphenyl dibromide was neutralized, washed with water and refined to obtain the product p-thiamphenyl dibromide with a yield of 95% and a purity of 99.2%.
Embodiment 2
[0029] First add p-thiamphenicol toluene to the reaction kettle, heat up to 120°C, add 0.5wt% catalyst copper chloride of p-thiamphenicol toluene, add bromine under stirring state, the mixture of p-thiamphenicol toluene and bromine The molar ratio was 1:10, and the heat preservation reaction was carried out for 15 hours to obtain the crude p-thiamphenicol dibromotoluene in liquid state.
[0030] Turn on the vacuum pump, vacuumize at -0.05MPa for 1.5 hours, remove the hydrobromic acid and unreacted bromine generated by the reaction under reduced pressure, and after the exhaustion is completed, put the liquid crude product p-thiamphenyl dibromotoluene into water under stirring , carry out water absorption treatment, and at the same time reduce the temperature and crystallize to obtain granular crude p-thiamphenicol dibromotoluene.
[0031] The solid crude product p-thiamphenyldibromotoluene is neutralized to neutral with sodium carbonate solution, washed with water after suction...
Embodiment 3
[0033] First add p-thiamphenicol toluene to the reaction kettle, heat up to 160°C, add 1.5wt% catalyst copper sulfate of p-thiamphenicol toluene, add bromine under stirring state, the mole of p-thiamphenicol toluene and bromine The ratio is 1:1.5, and the heat preservation reaction is carried out for 10 hours to obtain the liquid crude product p-thiamphenyldibromotoluene.
[0034] Turn on the vacuum pump, vacuumize at -0.06MPa for 4 hours, remove the hydrobromic acid and unreacted bromine generated by the reaction under reduced pressure, and after the exhaustion is completed, put the liquid crude product p-thiamphenyl dibromotoluene into water under stirring , carry out water absorption treatment, and at the same time reduce the temperature and crystallize to obtain granular crude p-thiamphenicol dibromotoluene.
[0035] The solid crude p-thiamphenyl dibromotoluene is neutralized to neutral with sodium hydroxide solution, washed with water after suction filtration, then heated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com