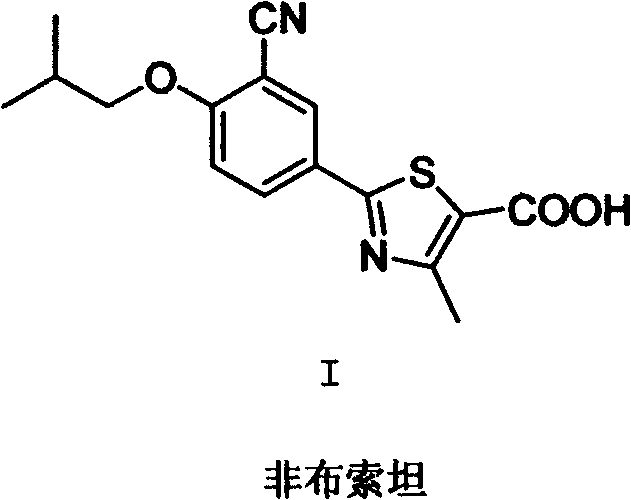
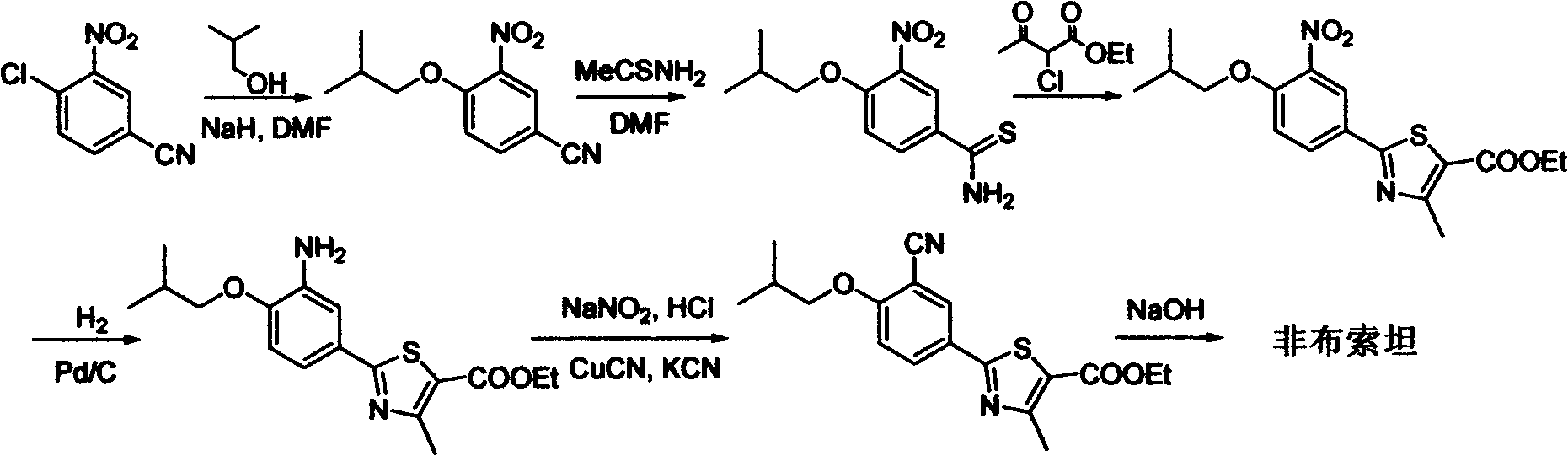
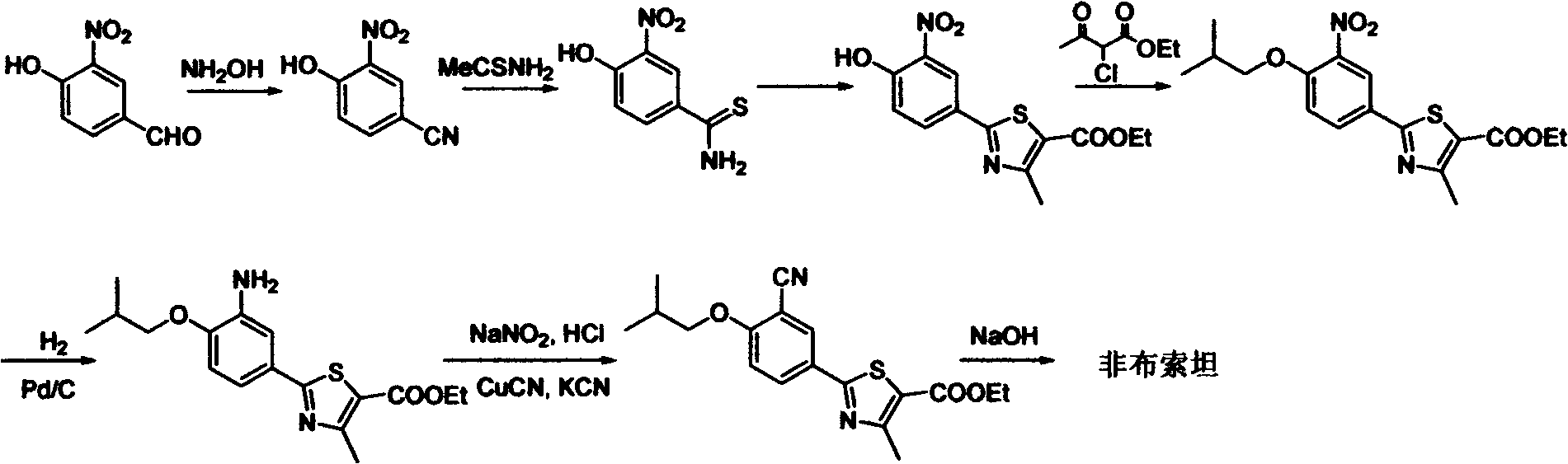
Febuxostat intermediate preparation method
A technology of febuxostat and intermediates, applied in the field of preparation of intermediates, can solve the problems of poor reaction selectivity, difficult industrialization, complicated operation, etc., and achieves the effects of low industrialization cost, short route and high atom utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of 3-cyano group-4-isobutoxy thiobenzamide
[0057] In a 1L reaction flask, add 4-isobutoxy-1,3-benzenedicarbonitrile (20g, 0.1mol), and then add 420mL of isopropanol. After the system is dissolved and clarified, cool the system down to 10°C with an ice bath Slowly add 51 g of 40% ammonium sulfide aqueous solution (containing 20.4 g of ammonium sulfide, 0.3 mol) dropwise in a dropwise manner. After the dropwise addition is completed, stir the reaction at 10° C. to 15° C. until the disappearance of the raw materials is monitored by TLC or HPLC. After about 20 hours of reaction, the reaction was stopped. Evaporate the solvent under reduced pressure, then add 200 mL of ethyl acetate and 100 mL of water, stir and separate the layers, extract the water layer with 200 mL of ethyl acetate twice, combine the organic phases, wash with 100 mL of distilled water for 3 times, add anhydrous sodium sulfate to the organic phase After drying, the solvent ...
Embodiment 2
[0059] Embodiment 2; The preparation of 3-cyano-4-isobutoxy thiobenzamide
[0060] In a 1L reaction flask, add 4-isobutoxy-1,3-benzenedicarbonitrile (20g, 0.1mol), and then add 420mL of isopropanol. After the system is dissolved and clarified, the system is heated to 50°C to Slowly add 51g of 20% ammonium sulfide aqueous solution (containing 10.2g of ammonium sulfide, 0.15mol) dropwise. After the dropwise addition is completed, stir the reaction at 45°C to 50°C until the raw materials are monitored by TLC or HPLC. After 6 hours, the reaction stopped. Evaporate the solvent under reduced pressure, then add 200 mL of ethyl acetate and 100 mL of water, stir and separate the layers, extract the water layer with 200 mL of ethyl acetate twice, combine the organic phases, wash with 100 mL of distilled water for 3 times, add anhydrous sodium sulfate to the organic phase After drying, the solvent was evaporated under reduced pressure to obtain a crude product in the form of a yellow so...
Embodiment 3
[0062] Embodiment 3: the preparation of 3-cyano group-4-isobutoxy thiobenzamide
[0063] In a 1L reaction flask, add 4-isobutoxy-1,3-phthalonitrile (20g, 0.1mol), then add ethylene glycol dimethyl ether 420mL, triethylamine 30.4g, after the system is dissolved and clarified , the system was heated up to 50°C, and 34g of 20% ammonium sulfide aqueous solution (containing 6.8g of ammonium sulfide, 0.10mol) was slowly added dropwise in a dropwise manner. Alternatively, HPLC monitors the completion of the reaction, and stops the reaction after about 5 hours of reaction. Evaporate the solvent under reduced pressure, then add 200 mL of ethyl acetate and 100 mL of water, stir and separate the layers, extract the water layer with 200 mL of ethyl acetate twice, combine the organic phases, wash with 100 mL of distilled water for 3 times, add anhydrous sodium sulfate to the organic phase After drying, the solvent was evaporated under reduced pressure to obtain the crude product as a yell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com