Helicobacter pylori immunodominance epitope peptide and preparation method and application thereof
A Helicobacter pylori, dominant epitope technology, applied in the field of medical biology, can solve problems such as self-reactive epitopes cannot be ruled out
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Step-overlap synthesis of 18 amino acid short peptides and preparation of mixed peptide library
[0046] Helicobacter pylori urease protein B subunit (UreB) has a full length of 569 amino acids, and its sequence is conserved among various strains. The recombinantly constructed Helicobacter pylori urease B subunit is a 1-569 polypeptide, which is derived from the 11637 international standard strain. The amino acid sequence of the B subunit of urease protein derived from Helicobacter pylori 11637 (number P69996) was retrieved from the UniProt protein database, and a short peptide of 18 amino acids was synthesized by stepping and overlapping from the first amino acid (synthesized with the assistance of Shanghai Jier Biochemical Company), a total of 93 strips (the last one is a 17 amino acid short peptide). The purity is greater than 70%. The synthetic peptide information is shown in Table 1. The synthesized peptides were dissolved in DMSO to a storage concen...
Embodiment 2
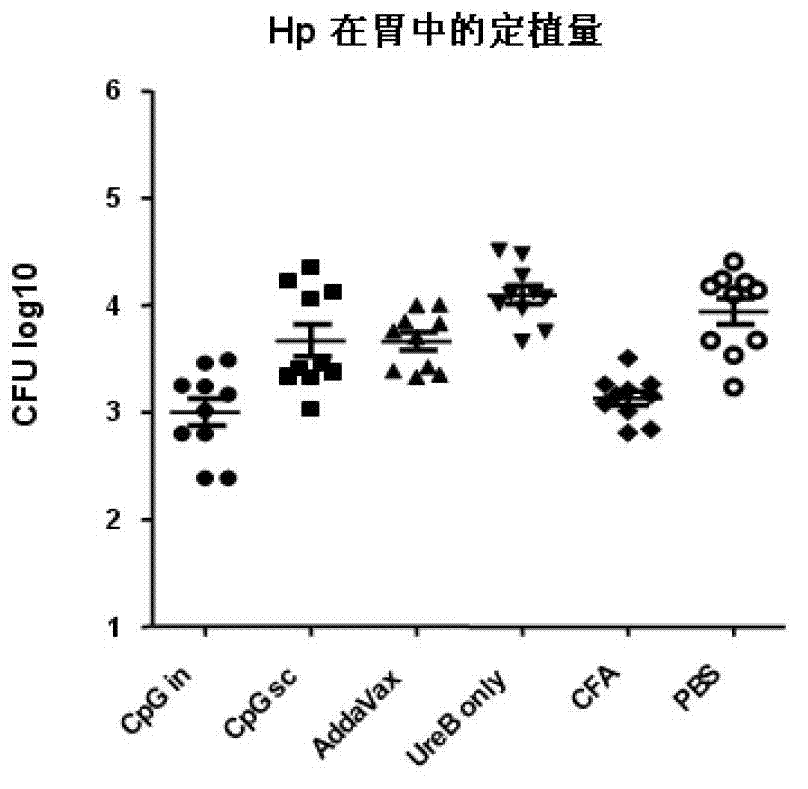
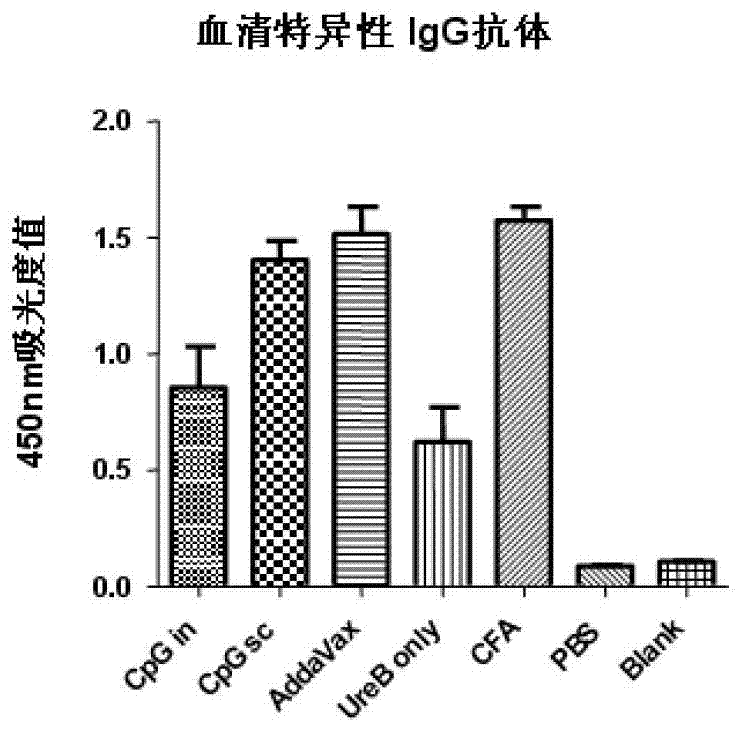
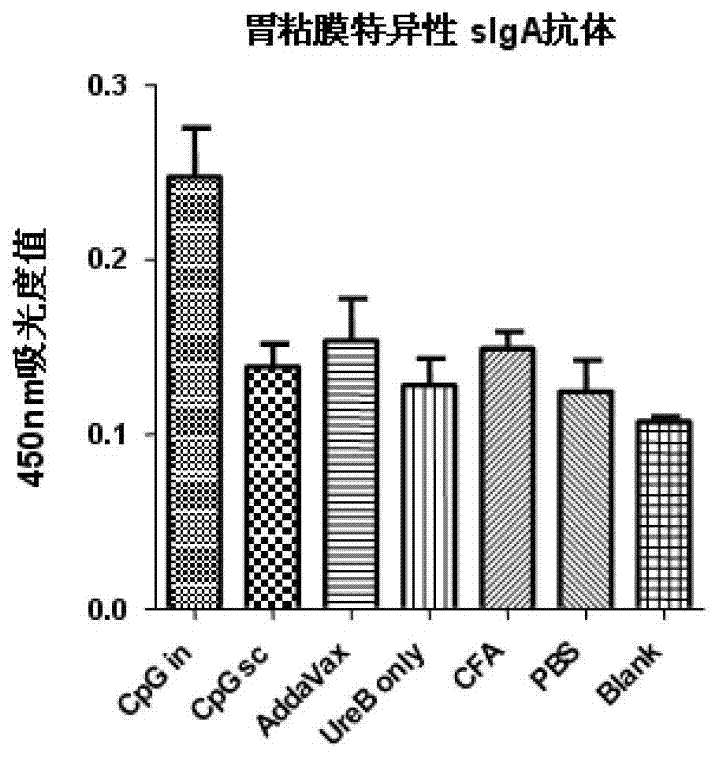
[0056] Example 2: Evaluation of immune effect and analysis of antibody response in different adjuvant groups
[0057] 1. Experimental protocol for animal immunization and challenge
[0058] Mice were immunized with rUreB antigen and different adjuvants. The specific immunization plan is as follows:
[0059] Experimental animals: BALB / c female mice aged 6-8 weeks.
[0060] Immunization methods: nasal drops, subcutaneous.
[0061] Immunization volume: intranasal, 8ul / monkey; subcutaneous, 200ul / monkey.
[0062] Experimental group:
[0063] ① rUreB (100ug / cause) + Freund's adjuvant (volume 1:1). Subcutaneous immunization 3 times (week 0, 2, 4).
[0064] ②rUreB (50ug / piece) + CpG (20ug / piece). Intranasal immunization 4 times (1st, 2nd, 3rd, 4th week).
[0065] ③rUreB (100ug / piece) + CpG (20ug / piece). Subcutaneous immunization 3 times (week 0, 2, 4).
[0066] ④ rUreB (100ug / piece) + AddaVax (volume 1:1). Subcutaneous immunization 3 times (week 0, 2, 4).
[0067] ⑤ rUr...
Embodiment 3
[0077] Example 3: Collection and Preservation of Lymphocytes in Mouse Spleen
[0078] The mouse spleen was isolated under aseptic conditions, ground, and made into a single cell suspension, and the buffy coat lymphocytes were collected after density gradient centrifugation of mouse lymphocyte separation medium. The isolated splenic lymphocytes were not used for in vitro culture, and the excess cells were adjusted to a cell concentration of 1×10 with cryopreservation solution. 7 / mL, add 1mL / tube into a cryopreservation tube, put it into a freezer box at -70°C overnight, and transfer it to liquid nitrogen for cryopreservation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com