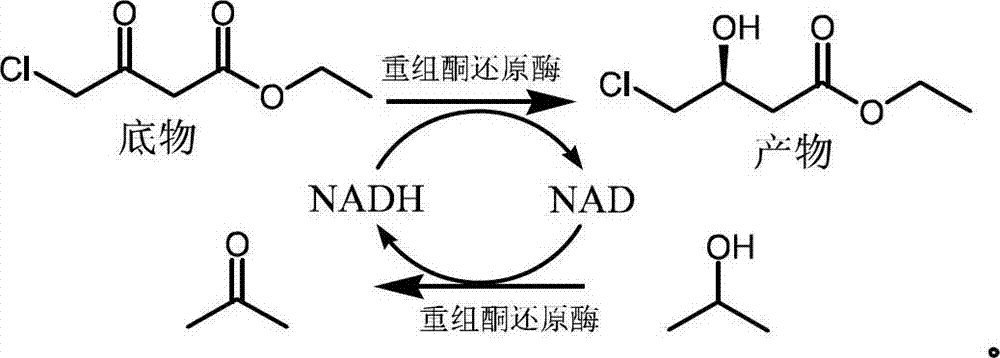
Biological preparation method of (S)-4-chloro-3-hydroxybutyrate ethyl
A technology for ethyl hydroxybutyrate and biological preparation, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of high dosage of enzymes and cofactors, poor safety, cumbersome operation, etc. Simple operation, high reaction efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 detects the HPLC / MS method of biotransformation reaction
[0021] Sample treatment: Take 50 μL of reaction solution at different time points, add 950 μL of methanol, dissolve, filter with 0.45 μm microporous membrane, and inject for detection. The injection volume is 1 μL. Chromatographic conditions: the chromatographic column is SB-C18 (2.1×50 mm, 3.5 μm), and the mobile phase is A water (0.1% HCOOH)-B acetonitrile. Flow rate 0.3mL / min, 0-3min 30%B. Mass spectrometry conditions: dry gas flow rate 12L / min, sheath gas pressure 40PSI, dry gas temperature 350°C, capillary voltage 3500V, detection mode is positive ion mode, detected ions 165.1, 167.1, 169.1. (S)-Ethyl 4-chloro-3-hydroxybutyrate has a retention time of 1.1 min, and ethyl 4-chloro-3-oxobutyrate has a retention time of 1.6 min.
Embodiment 2
[0022] Example 2 Gas Chromatographic Method for Detecting Biotransformation Reactions
[0023] Sample treatment: Take 200 μL of the reaction solution at different time points, add an equal volume of ethyl acetate to extract twice, take the organic phase and filter it with a 0.45 μm microporous membrane, and inject a sample for detection, the injection volume is 1 μL. Chromatographic conditions: CP-chirasil-dex CB 25*0.32mm*0.25μm column, carrier gas: nitrogen, inlet temperature: 180°C, detector temperature: 280°C, split ratio: 30:1, temperature program: The initial temperature is 70°C, the temperature is raised to 110°C at 10°C / min, and kept for 6 minutes, then the temperature is raised to 200°C at 40°C / min, and kept for 1.5 minutes. The retention time of ethyl (S)-4-chloro-3-hydroxybutyrate is 7.6min, and the retention time of ethyl 4-chloro-3-oxobutyrate is 3.7min.
Embodiment 3
[0024] Example 3 Preparation of recombinant ketoreductase
[0025] Inoculate a single colony of recombinant Escherichia coli containing the ketoreductase gene from a glycerol tube or transformation plate into 4 mL of liquid LB medium containing ampicillin resistance and activate overnight (37° C., 200 rpm). Transfer 100mL liquid LB medium containing ampicillin resistance from the overnight culture at 1 / 100 inoculum size, culture at 37°C, 200rpm shaking until OD 600 When the value reaches 0.6-0.8, add IPTG and continue culturing overnight at 30°C. The cells were collected by centrifugation, and suspended in 10 mL of phosphate buffer (2 mM, pH 7.0). The cell suspension was ultrasonically disrupted in an ice bath for 10 minutes, centrifuged, the supernatant was pre-frozen overnight, and freeze-dried for 24h-48h to obtain ketoreductase KRED in the form of freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com