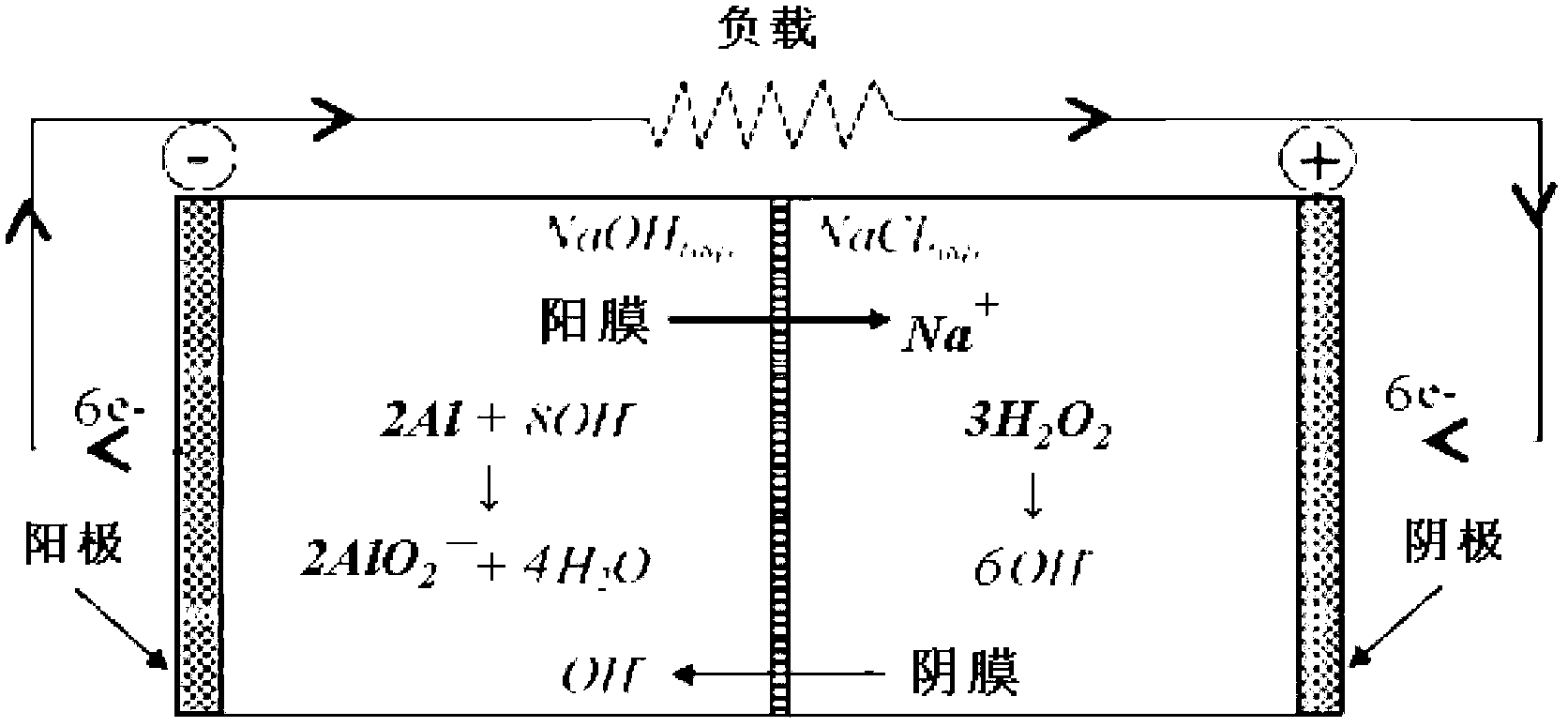
High-energy low-consumption Al-H2O2 semi-fuel cell
A semi-fuel cell and high-energy technology, which is applied in the direction of fuel cell half-cells and primary battery-type half-cells, can solve the problem of reducing the ion conductivity and service life of ion exchange membranes, reducing the catalytic performance of cathode catalysts, and reducing the high temperature of batteries. Specific energy advantages and other issues, to achieve the effect of increasing service life, reducing decomposition rate, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
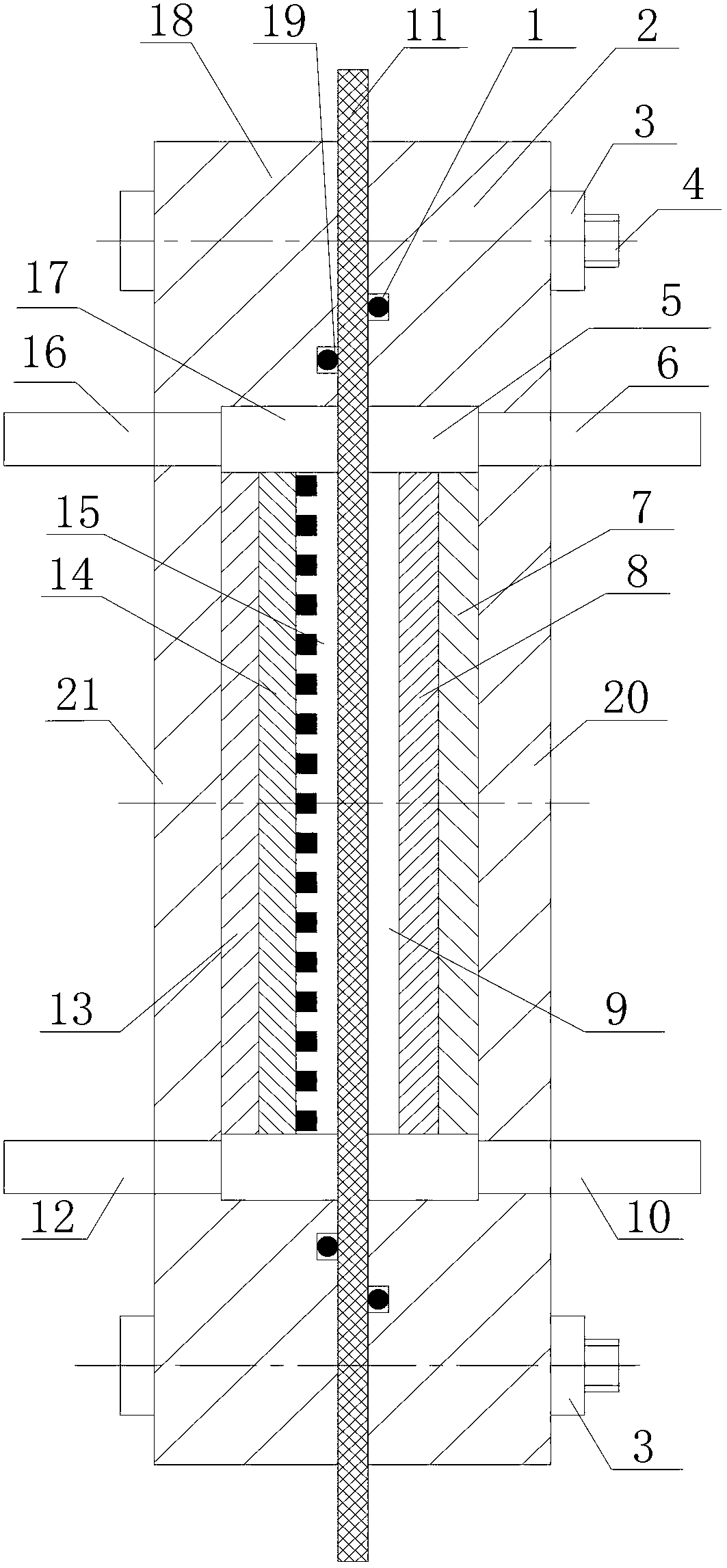
[0025] High energy and low consumption Al-H of the present invention 2 o 2 The production process of the half fuel cell: such as figure 1 Shown:
[0026] The square 3240 epoxy phenolic resin plate with a thickness greater than 10mm is used as the material for making the anode part 21 and the cathode part 20. The anode part is processed with an anode groove 17 at the center of one side as the anode casing 18, and the bottom surface of the anode groove is formed with two holes. Holes are respectively used as anolyte inlet 12 and anolyte outlet 16, and a through hole is processed near the four corners of the anode casing, and an annular groove is made on the unsealed end surface of the anode casing as the anode sealing groove, and the anode sealing groove An anode sealing ring 19 is placed in the anode groove. From left to right in the anode groove are the anode current collector plate 13 made of nickel plate and the anode plate 14 made of aluminum alloy. The gap between the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com