Preparation method of hydroxyl-terminated hyperbranched polyurethane
A technology of hydroxyl-terminated hyperbranching and polyurethane, which is applied in the field of hyperbranched polymers, can solve the problems that it is difficult to obtain high-purity hyperbranched polymers, the purity of hyperbranched molecules is difficult to guarantee, and the number of branch growth needs to be increased. Improved stability and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
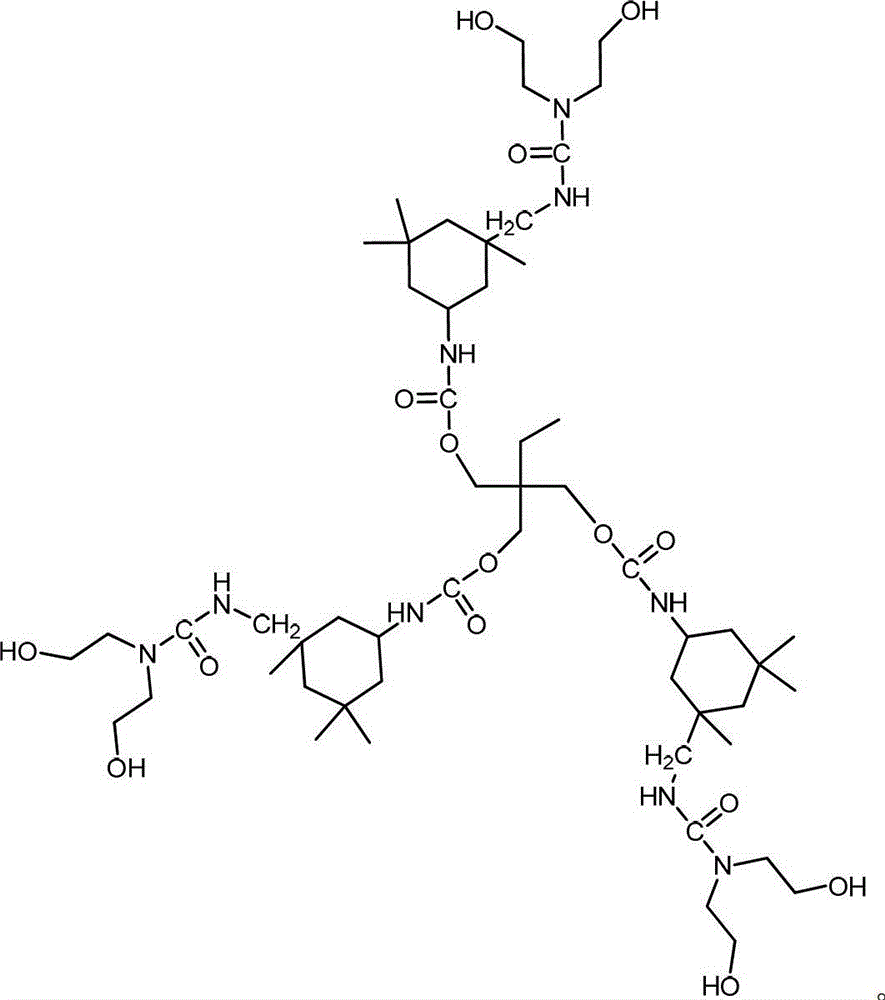
[0022] The target molecular structure formula of the first-generation hydroxyl-terminated hyperbranched polyurethane is as follows:
[0023]
[0024] The synthesis operation steps are as follows:
[0025] Take 4 grams of trimethylolpropane (TMP) and dissolve it in 20 grams of N,N-dimethylformamide (DMF) to obtain a hydroxyl-terminated small molecule polyol solution. Get 19.88 grams of isophorone diisocyanate (IPDI) and heat it to 85°C, add it to the hydroxyl-terminated small molecule polyol solution and stir for 6 hours to obtain the first generation of hyperbranched prepolymer a 1 . 43.88 grams of the first generation hyperbranched prepolymer a 1 and 23.5 grams of diethanolamine were cooled to 10°C, and the heat preservation and blending reaction was carried out for 6 hours. The obtained product was separated and purified with acetone aqueous solution with a mass fraction of 10%, and then vacuum-dried to obtain the first-generation hydroxyl-terminated hyperbranched polyu...
Embodiment 2
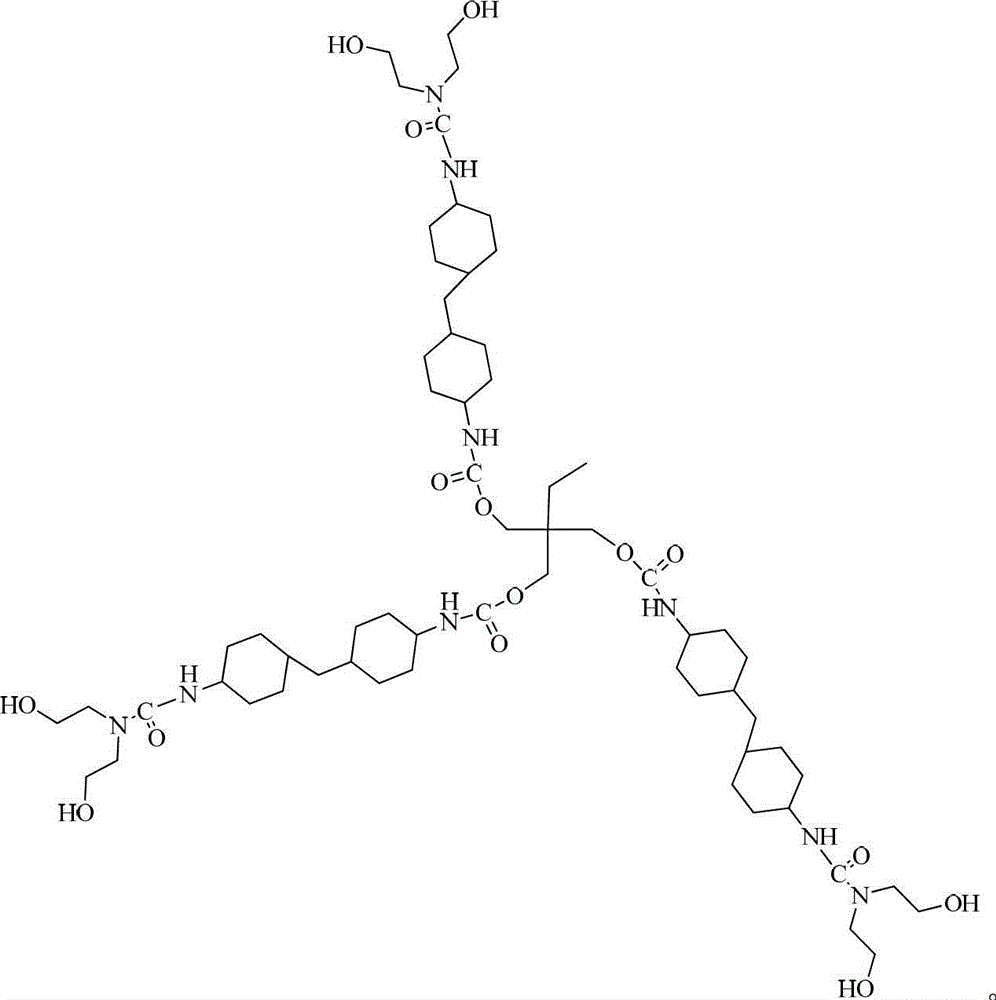
[0028] The target molecular structure formula of the first-generation hydroxyl-terminated hyperbranched polyurethane is as follows:
[0029]
[0030] The synthesis operation steps are as follows:
[0031] Dissolve 4 g of trimethylolpropane (TMP) in 20 g of N,N-dimethylacetamide (DMAC) to obtain a hydroxyl-terminated small molecule polyol solution. Heat 20.46 grams of 4,4'-dicyclohexylmethane diisocyanate (HMDI) to 90°C, add it into the hydroxyl-terminated small molecule polyol solution and stir for 4 hours to obtain the first-generation hyperbranched prepolymer a 1 . 44.46 grams of the first generation hyperbranched prepolymer a 1 and 18.5 grams of diethanolamine were cooled to 15°C, and the heat preservation, blending and stirring reaction was carried out for 6 hours, and the obtained product was separated and purified with water and then vacuum-dried to obtain the first-generation hydroxyl-terminated hyperbranched polyurethane A of a colorless transparent solid 1 .
Embodiment 3
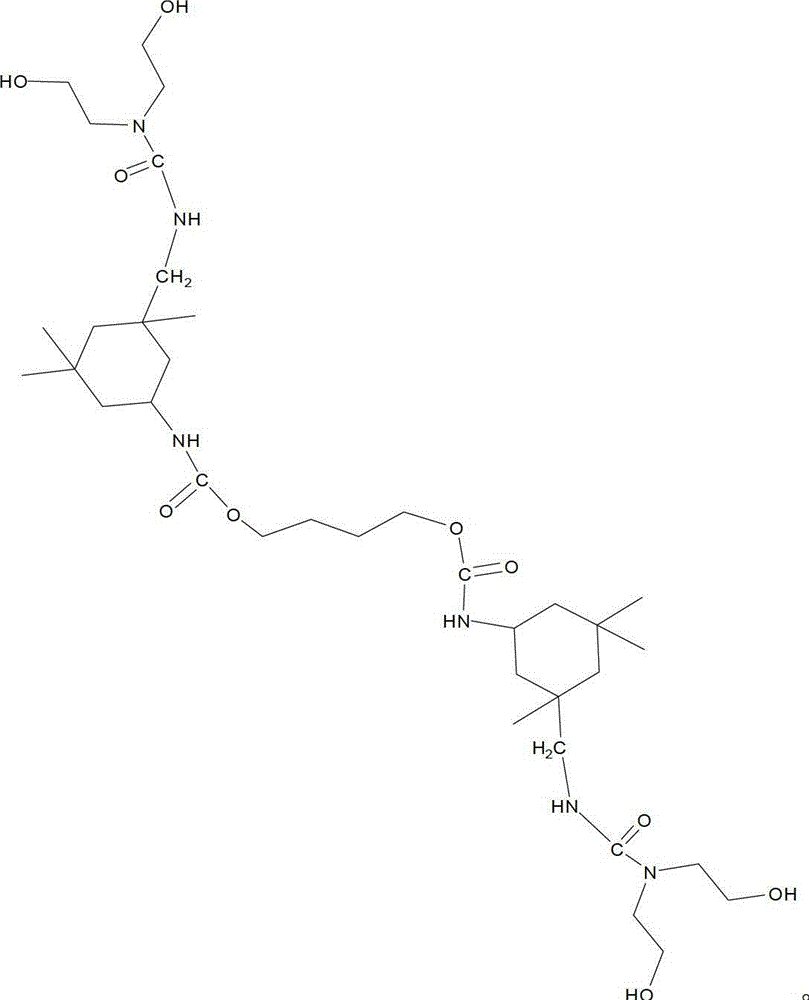
[0033] The target molecular structure formula of the first-generation hydroxyl-terminated hyperbranched polyurethane is as follows:
[0034]
[0035] The synthesis operation steps are as follows:
[0036] Take 3 grams of 1,4-butanediol (BDO) and dissolve it in 20 grams of N,N-dimethylformamide (DMF) to obtain a hydroxyl-terminated small molecule polyol solution. Heat 11.78 grams of isophorone diisocyanate (IPDI) to 80°C, add the hydroxyl-terminated small molecule polyol solution and stir for 6 hours to obtain the first-generation hyperbranched prepolymer a 1 . 34.78 grams of the first generation hyperbranched prepolymer a 1 and 17 grams of diethanolamine were cooled to 10°C, and the heat preservation and blending reaction was carried out for 6 hours. The obtained product was separated and purified with a 5% ether aqueous solution and then vacuum-dried to obtain the first-generation hydroxyl-terminated hyperbranched polyurethane A of a colorless transparent solid. 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com