Self-reinforced bi-crosslinking hyaluronic acid hydrogel and preparation method thereof
A hyaluronic acid and hydrogel technology, applied in the field of biomedical materials and tissue engineering, can solve the problems of lack of binding and controlled release of growth factors, non-degradable or poor degradation performance, biocompatibility problems, etc. The effect of regeneration and repair, improving mechanical properties and high water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
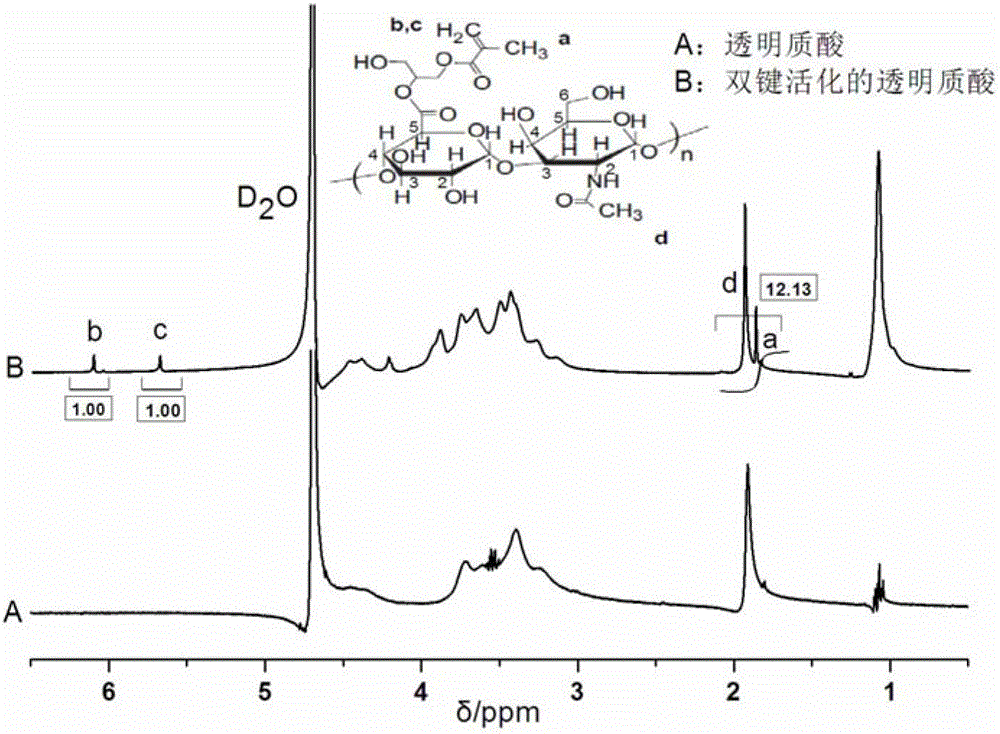
[0041] (1) Add hyaluronic acid into deionized water to prepare a hyaluronic acid aqueous solution with a mass concentration of 0.002g / mL. Under magnetic stirring, add triethylamine (the molar quantity added is 20% of all the hydroxyl moles of hyaluronic acid), add tetrabutylammonium bromide after reacting for 1h (the molar quantity added is all the hydroxyl moles of hyaluronic acid 20% of the number of moles of hyaluronic acid), after reacting for 1 hour, add glycidyl methacrylate to the system (the molar amount added is 50 times the number of moles of carboxyl groups of hyaluronic acid), react at 20°C for 2 days, and keep warm at 60°C 1h, then dialyzed in 0.1mol / L sodium chloride solution and deionized water for 7d, and finally freeze-dried for 16h to obtain double bond-activated hyaluronic acid with a double bond substitution degree of 32.86%. like figure 1 Shown are hyaluronic acid and double bond activated hyaluronic acid 1 H NMR spectrum. like figure 1 As shown in the...
Embodiment 2
[0046] (1) Add hyaluronic acid into deionized water to prepare a hyaluronic acid aqueous solution with a mass concentration of 0.002g / mL. Under magnetic stirring, add triethylamine (the molar quantity added is 20% of all the hydroxyl moles of hyaluronic acid), add tetrabutylammonium bromide after reacting for 1h (the molar quantity added is all the hydroxyl moles of hyaluronic acid 20% of the number of moles of hyaluronic acid), after reacting for 1 hour, add glycidyl methacrylate to the system (the molar amount added is 10 times the number of moles of carboxyl groups of hyaluronic acid), react at 30°C for 2 days, and keep warm at 60°C 1h, then dialyzed in 0.1mol / L sodium chloride solution and deionized water for 7d, and finally freeze-dried for 12h to obtain double bond-activated hyaluronic acid with a double bond substitution degree of 2.8%.
[0047] (2) Dissolve hyaluronic acid in 0.2 mol / L sodium hydroxide solution to prepare a hyaluronic acid aqueous solution with a mass ...
Embodiment 3
[0050] (1) Add hyaluronic acid into deionized water to prepare a hyaluronic acid aqueous solution with a mass concentration of 0.002g / mL. Under magnetic stirring, add triethylamine (the molar quantity added is 20% of all the hydroxyl moles of hyaluronic acid), add tetrabutylammonium bromide after reacting for 1h (the molar quantity added is all the hydroxyl moles of hyaluronic acid 20% of the number of moles of hyaluronic acid), after reacting for 1 hour, add glycidyl methacrylate to the system (the molar amount added is 100 times the number of moles of carboxyl groups of hyaluronic acid), react at 25°C for 2 days, and keep warm at 60°C 1h, then dialyzed in 0.1mol / L sodium chloride solution and deionized water for 7d, and finally freeze-dried for 24h to obtain double bond-activated hyaluronic acid with a double bond substitution degree of 65.0%.
[0051] (2) Dissolve hyaluronic acid in 0.2 mol / L sodium hydroxide solution to prepare a hyaluronic acid aqueous solution with a mas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com