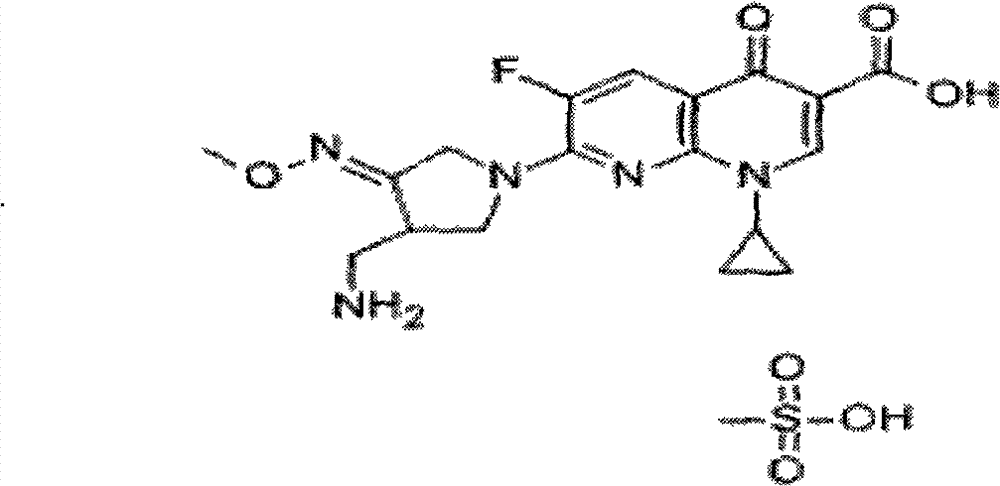
Preparation method of gemifloxacin mesylate medicinal composition
A technology of gemifloxacin mesylate and a composition, which is applied in the field of preparation of pharmaceutical compositions, can solve the problems of uneven granulation, many fine powders and the like, and achieves uniform particles, large drug loading, and difference in tablet weight. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The three methods of wet granulation, fluidized bed granulation and wet pre-granulation combined with fluidized bed granulation were compared, as follows:
[0046] Scheme A: wet granulation steps:
[0047]Add 426.39g of gemifloxacin mesylate to the granulation equipment together with 49.01g of microcrystalline cellulose and 26.00g of cross-linked polyvinylpyrrolidone after crushing and sieving, stir and mix, and add 14.70g of polyvinylpyrrolidone 5% w / w Aqueous solution of 80-120 r / min stirring and 150-200 r / min shearing force to make granules, after drying, granulate and add 3.90g magnesium stearate to mix and tablet , weighing.
[0048] Scheme B: Fluidized bed granulation steps:
[0049] Put 426.39g of gemifloxacin mesylate into the fluidized bed after crushing and sieving treatment, together with 49.01g of microcrystalline cellulose and 26.00g of cross-linked polyvinylpyrrolidone, and mix with the bottom air blower, and use 0.80mm-1.2 mm spray gun into 14.70g poly...
Embodiment 2
[0061] Prepare gemifloxacin mesylate tablets according to the method of scheme C wet pre-granulation in Example 1 combined with fluidized bed granulation, the type of binder used is specifically shown in Table 3, wherein the wet pre-granulation step and fluidized bed The type of binder used in the granulation step was consistent.
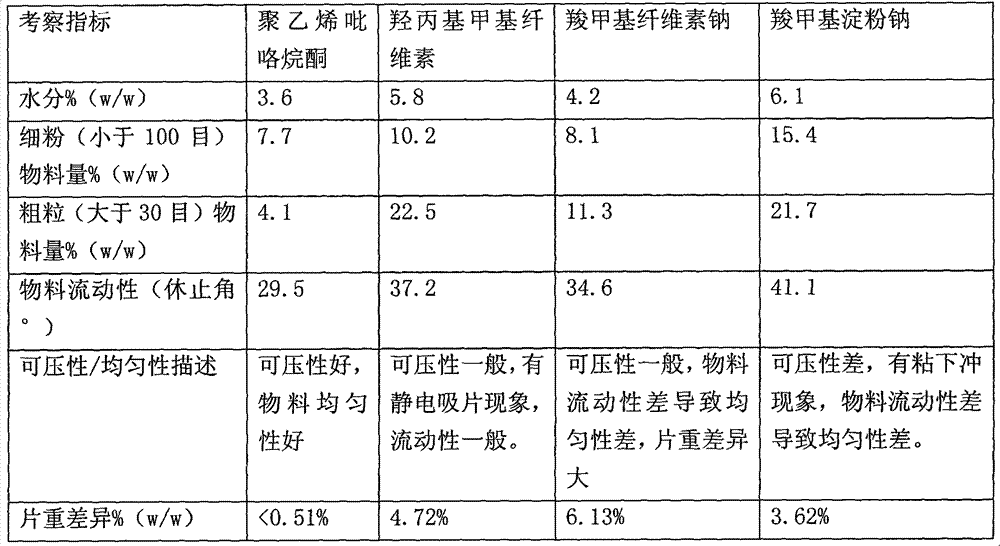
[0062] The influence of different binders (polyvinylpyrrolidone, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, sodium carboxymethylstarch) on wet pregranulation combined with fluidized bed granulation is shown in Table 3 below. Show:
[0063] Table 3: Effect of different binders on wet pre-granulation combined with fluidized bed granulation process
[0064]
[0065] It can be seen from Table 3 that in the process of wet pre-granulation combined with fluidized bed granulation, the gemifloxacin mesylate granules prepared by selecting polyvinylpyrrolidone and hydroxypropyl methylcellulose as binders have good uniformity and can be c...
Embodiment 3
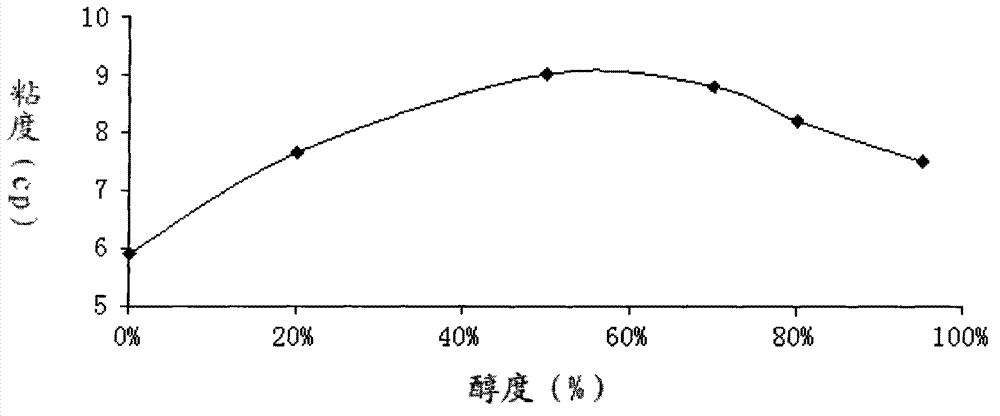
[0067] Wet pre-granulation: Put 426.39g of gemifloxacin mesylate through a 60-mesh sieve and 49.01g of microcrystalline cellulose for pre-mixing in a high-shear one-step granulator, and then add 150ml of aqueous solution of polyvinylpyrrolidone in groups A-E Perform primary granulation. Granules are made under two forces of 80-120 rpm stirring and 150-200 rpm shearing. The time of wet pre-granulation is 20-240 seconds, the temperature is 60°C, and the pressure is 1.5p.
[0068] Fluidized bed re-granulation: Add the material obtained by wet pre-granulation into the fluidized bed, and spray 150ml of aqueous solution of polyvinylpyrrolidone in groups A-E respectively under the state of atomization, and control the injection speed to 1-100g / min, atomize The pressure is 0.4-1.0mbar, the temperature of the material is 30-60°C, then granulate and dry, add 26.00g cross-linked polyvinylpyrrolidone and 3.90g magnesium stearate to the dried granules, mix well, press into tablets, and wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com