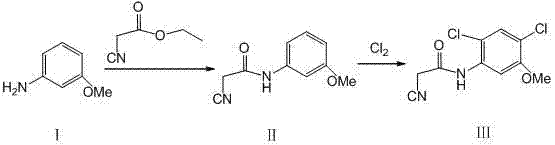
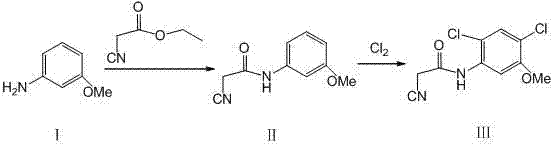
Synthesis method of 2-cyano-N-(2, 4-dichloro-5-methoxyphenyl) acetamide
A technology of methoxyphenyl and synthesis method, which is applied in the field of compound chlorination synthesis, can solve the problems of cumbersome post-treatment, poor selectivity, and difficult process, and achieve the effects of cost control, mild reaction conditions, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Preparation of II:
[0013] Add m-methoxyaniline 123g and ethyl cyanoacetate 180g to a 1000mL four-necked flask equipped with mechanical stirring, a thermometer and a condenser, and start stirring; the temperature is raised to 200°C, and the temperature is kept for 4h; the reaction solution is lowered to room temperature, and there are The solid was precipitated and filtered; the filter cake was washed with solvent to obtain II181.7g, the yield was 95.6%.
[0014] Preparation of III:
[0015] Add Ⅱ190.2g and 1250mL chloroform to a 1000mL jacketed kettle equipped with mechanical stirring, thermometer, air pipe and exhaust gas absorption, turn on stirring, and cool down to -5℃; 255.6g was introduced in about 4h; the reaction was complete, the system was purged with nitrogen, and the material was filtered; the filter cake was washed with water to obtain 242.6g of the target product III crude product; the solvent was recrystallized and dried to obtain 230.5g of solid produ...
Embodiment 2
[0017] Preparation of II:
[0018] Add m-methoxyaniline 123g and ethyl cyanoacetate 339g to a 1000mL four-necked flask equipped with mechanical stirring, a thermometer and a condenser, and start stirring; the temperature is raised to 180°C, and the temperature is kept for 4h; the reaction solution is lowered to room temperature, and there are The solid was precipitated and filtered; the filter cake was washed with solvent to obtain II 182.4 g with a yield of 96%.
[0019] Preparation of III:
[0020] In a 1000mL jacketed kettle equipped with mechanical stirring, thermometer, gas pipe and exhaust gas absorption, add Ⅱ190.2g, 1,2-dichloroethane 1250mL, turn on stirring, cool down to 0℃; pass chlorine gas, and control the temperature at 0 ~5°C, 284g was introduced in about 4h; the reaction was complete, the system was purged with nitrogen, and the material was filtered; the filter cake was washed with water to obtain 231.7g of the target product III crude product; the solvent wa...
Embodiment 3
[0022] Preparation of II:
[0023] Add m-methoxyaniline 123g and ethyl cyanoacetate 226g to a 1000mL four-necked flask equipped with mechanical stirring, a thermometer and a condenser, and start stirring; heat up to 150°C, and keep the temperature for 6h; lower the reaction solution to room temperature, and there are The solid was precipitated and filtered; the filter cake was washed with solvent to obtain II 171 g with a yield of 90%.
[0024] Preparation of III:
[0025] Add Ⅱ190.2g and 1250mL ethyl acetate into a 1000mL jacketed kettle equipped with mechanical stirring, thermometer, gas pipe and exhaust gas absorption, and start stirring; pass chlorine gas, control the temperature at 10-20℃, and pass 284g in about 4h; The reaction was completed, the system was purged with nitrogen, and the material was filtered; the filter cake was washed with water to obtain 232 g of the target product III crude product; the solvent was recrystallized and dried to obtain 215 g of solid pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com