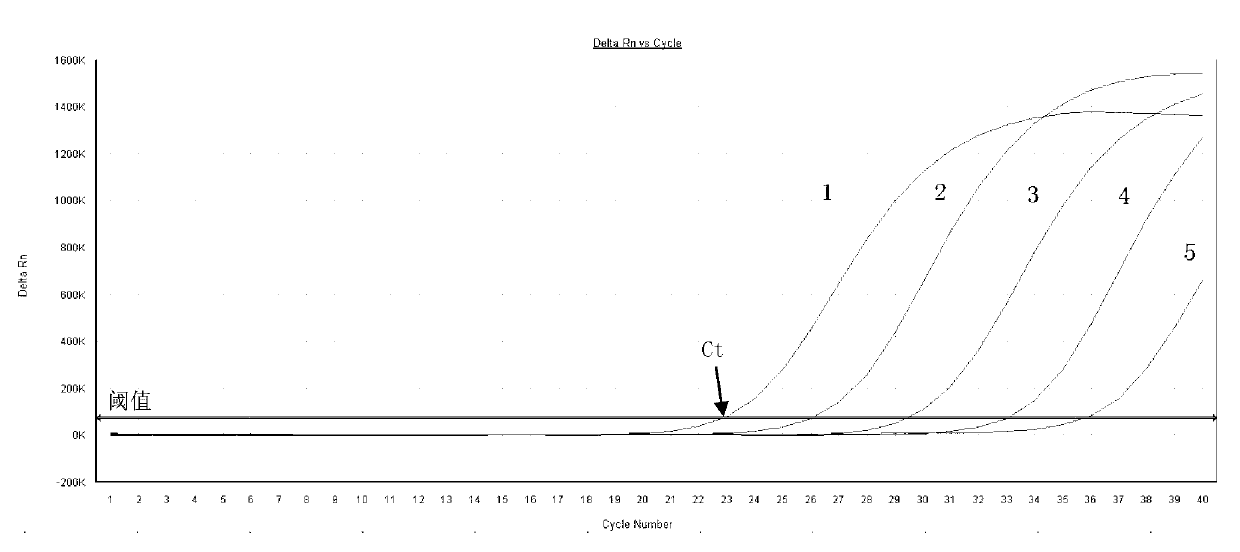
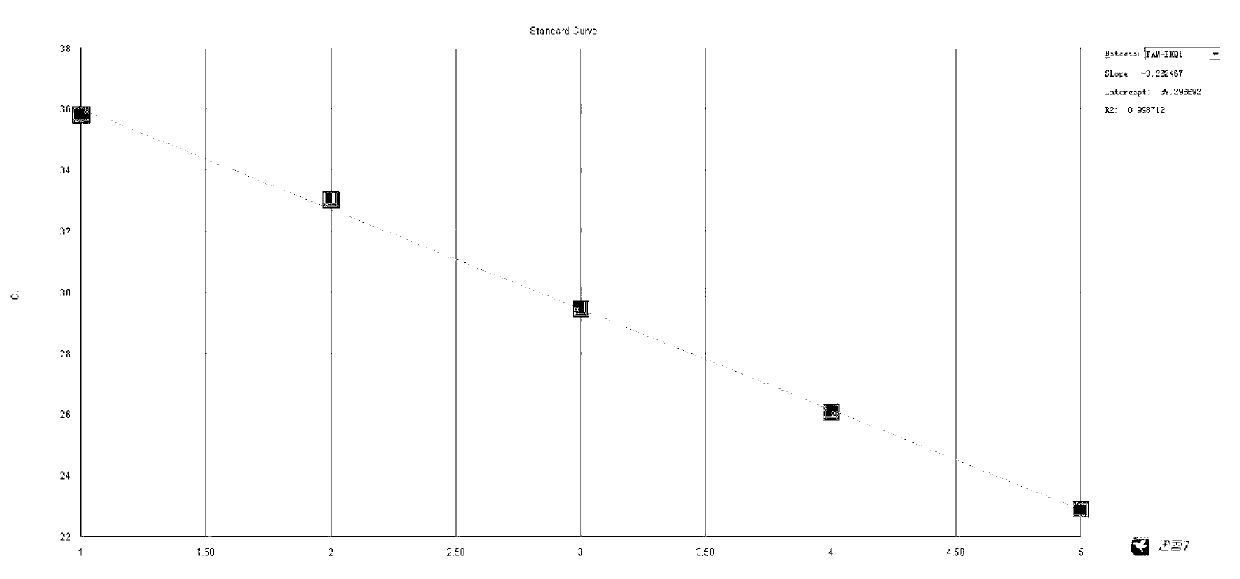
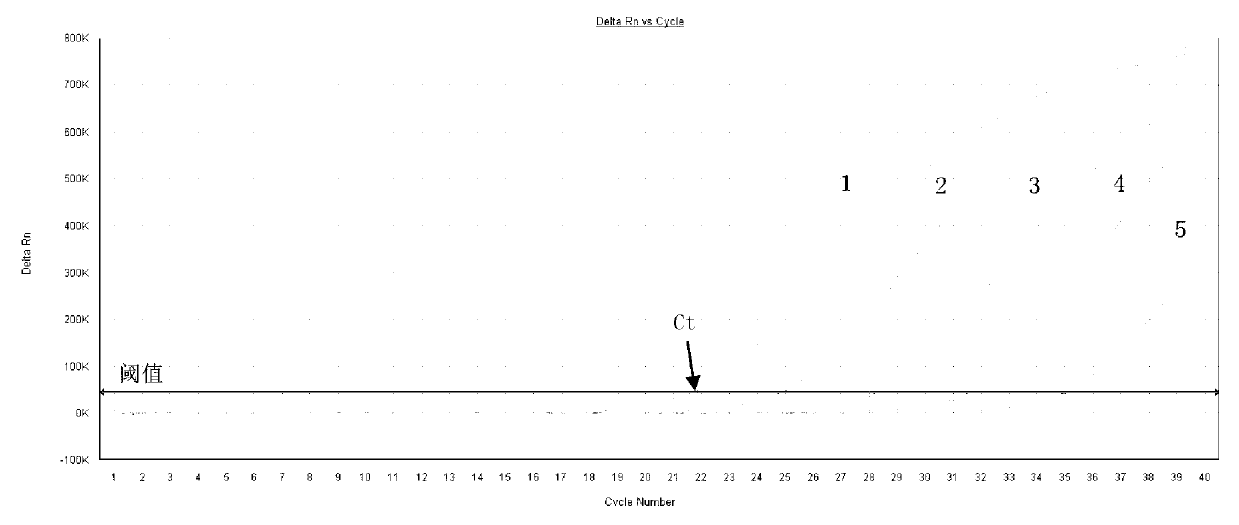
Dual fluorescence quantitative PCR (polymerase chain reaction) detection method and detection kit for clostridium difficile enterotoxin A and B
A detection method and clostridial intestinal technology, applied in the field of biomedicine, can solve the problems of missed diagnosis of patients, increased reports of serious infections and outbreaks, inability to distinguish toxigenic strains of Clostridium difficile from non-toxigenic strains, etc. High sex and specificity, reducing the effect of serious infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Experimental Materials
[0023] 1.1 Bacterial strains and samples to be tested
[0024] The positive nucleic acids of enteric adenovirus 40 / 41, astrovirus and saruvirus (all identified by gene sequencing) were obtained from the Jiangsu Provincial Center for Disease Control and Prevention.
[0025] The samples to be tested came from the stool samples of patients in Jiangsu Province recently, and the samples were collected and transported to the laboratory with ice.
[0026] 1.2 Primers and probes
[0027] Download Clostridium difficile enterotoxin A and B gene sequences from around the world from the NCBI gene bank in the United States, compare their homology, and design specific primers and Taqman fluorescent probes in the conserved gene regions corresponding to the virus genome. The specific sequences as follows:
[0028] Specific primers and probes for the enterotoxin A gene:
[0029] Upstream primer Toxa-FP: TTTTATGCCAGAAGCTCGCT (SEQ ID No: 1)
[0030] Downs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com