Camptothecin medical lipidosome composition and preparation method thereof
A technology of liposome composition and camptothecin, applied in drug combination, liposome delivery, pharmaceutical formulation, etc., can solve problems such as difficult to reach tumor sites, achieve good clinical value, stabilize physiological pH, and reduce toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
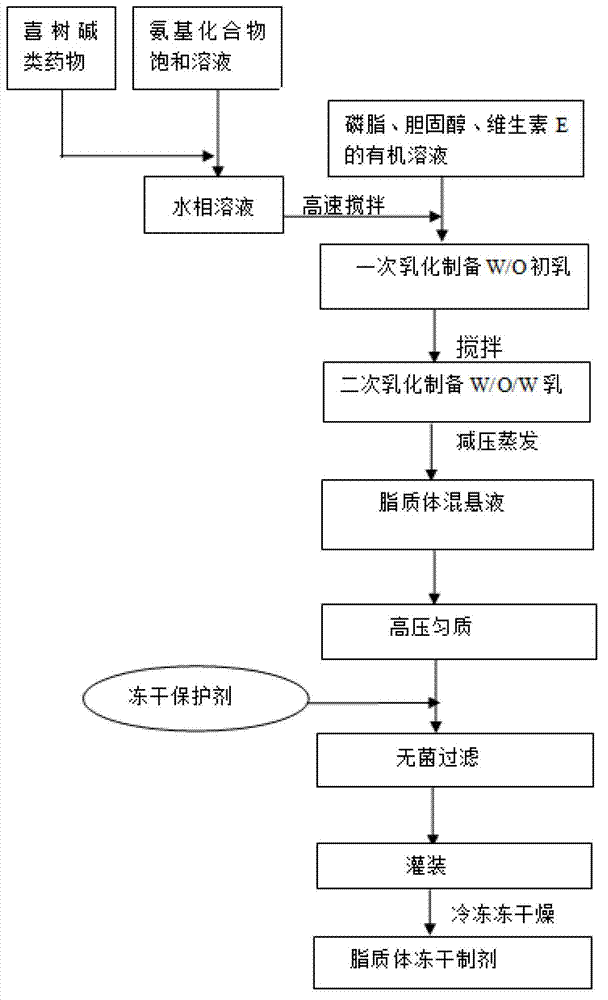
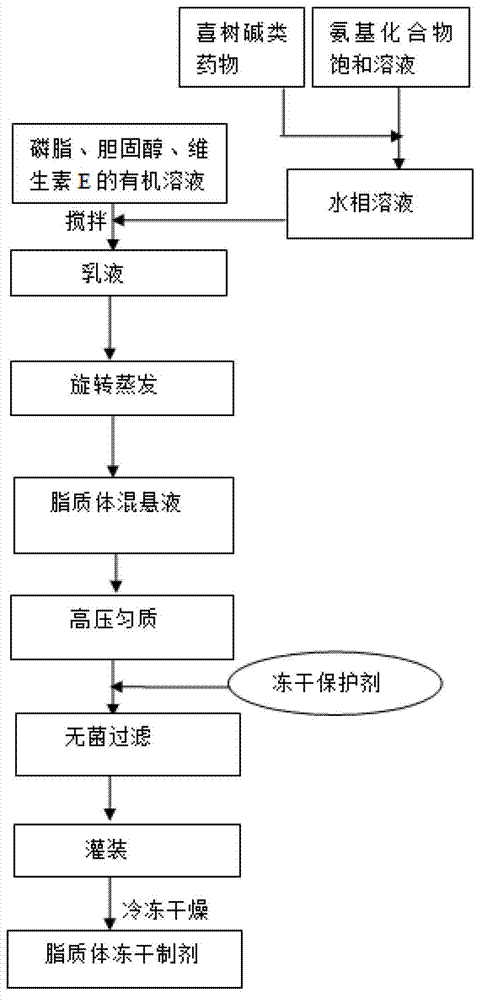
[0037] Embodiment 1, adopt double emulsification method to prepare camptothecin class drug liposome composition
[0038] Prepare camptothecin class drug liposome composition, adopt double emulsification method, comprise the following steps:
[0039] (1) 56g of soybean lecithin, 8g of cholesterol and 2.8g of vitamin E are co-dissolved in an appropriate amount of dichloromethane solvent as the organic phase;
[0040] (2) Add 5 g of 10-hydroxycamptothecin to 25 mL of saturated meglumine solution, stir at 50°C until clear, and cool to room temperature as the water phase;
[0041] (3) Mix the solutions of step (1) and step (2) according to the volume ratio of the organic phase and the aqueous phase at 2:1, and stir at 8000 rpm for 1 minute at high speed to prepare W / O colostrum;
[0042] (4) Quickly pour W / O colostrum into 450ml water for injection for secondary emulsification, and stir at 5000 rpm for 10 minutes;
[0043] (5) On a rotary evaporator, remove the organic solvent by...
Embodiment 2
[0047] Embodiment 2, adopt double emulsification method to prepare camptothecin class drug liposome composition
[0048] Prepare camptothecin class drug liposome composition, adopt double emulsification method, comprise the following steps:
[0049] (1) 72g of hydrogenated soybean lecithin, 33g of cholesterol and 1.0g of vitamin E are co-dissolved in an appropriate amount of dichloromethane solvent as the organic phase;
[0050] (2) Add 9.0 g of 10-hydroxycamptothecin to 50 mL of saturated meglumine aqueous solution, stir at 50°C until clear, and cool to room temperature as the water phase;
[0051] (3) Mix the solutions of step (1) and step (2) according to the volume ratio of the organic phase and the aqueous phase at 10:1, and stir at a high speed of 14000 rpm for 1 minute to prepare W / O colostrum;
[0052] (4) Quickly pour W / O colostrum into 4L water for injection for secondary emulsification, and stir at 5000 rpm for 30 minutes;
[0053] (5) On a rotary evaporator, remo...
Embodiment 3
[0057] Embodiment 3, adopt double emulsification method to prepare camptothecin drug liposome composition
[0058] Prepare camptothecin class drug liposome composition, adopt double emulsification method, comprise the following steps:
[0059] (1) 48g egg yolk lecithin, 15g cholesterol and 2.5g vitamin E are co-dissolved in an appropriate amount of dichloromethane solvent as the organic phase;
[0060] (2) Add 5 g of irinotecan to 20 mL of saturated meglumine aqueous solution, stir at 50°C until clear, and cool to room temperature as the water phase;
[0061] (3) Mix the solutions of step (1) and step (2) according to the volume ratio of the organic phase and the aqueous phase at 5:1, and stir at 8000 rpm for 1 minute at high speed to prepare W / O colostrum;
[0062] (4) Quickly pour W / O colostrum into 450mL water for injection for secondary emulsification, and stir at 5000 rpm for 10 minutes;
[0063] (5) On a rotary evaporator, remove the organic solvent by evaporating unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com