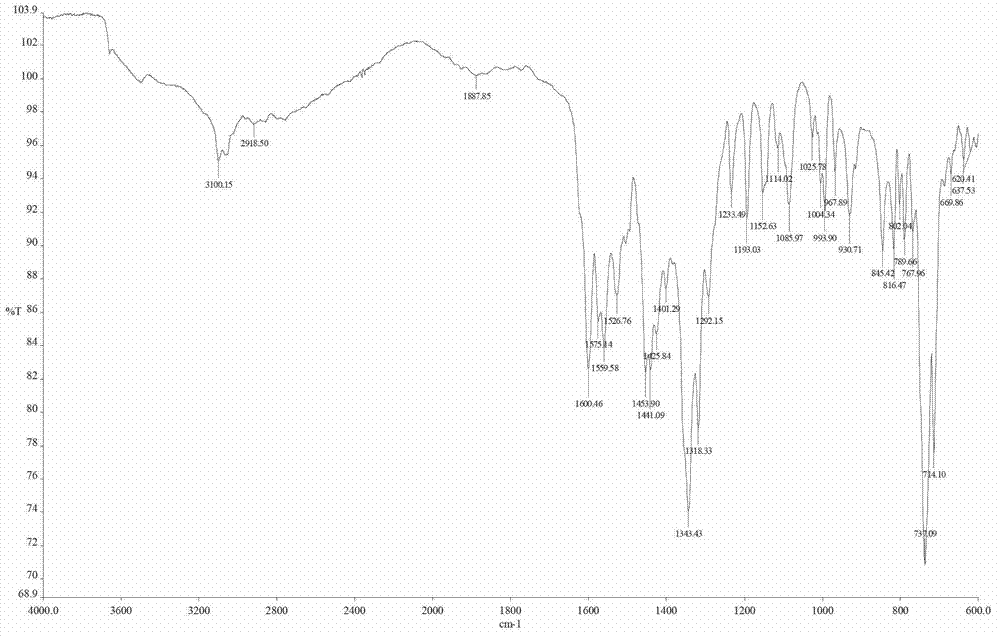
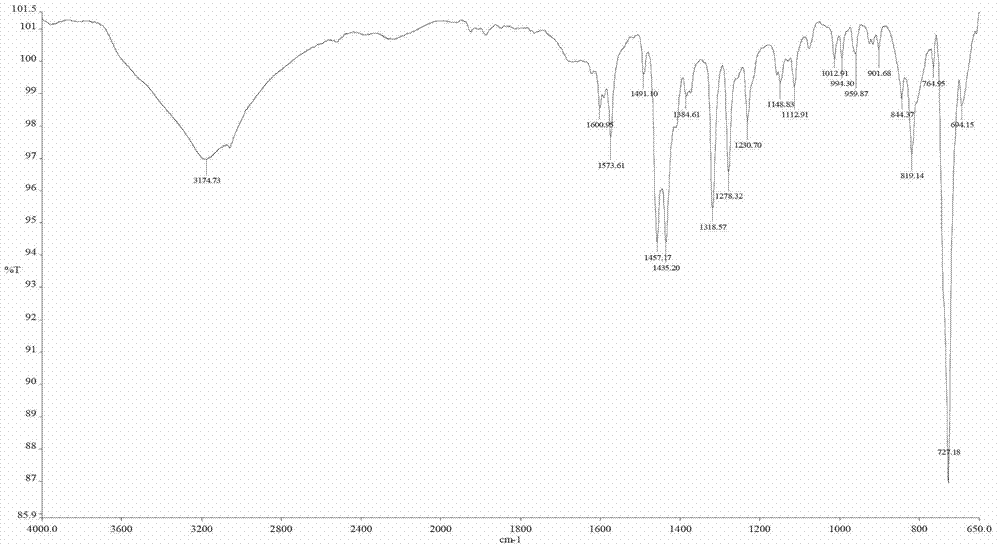
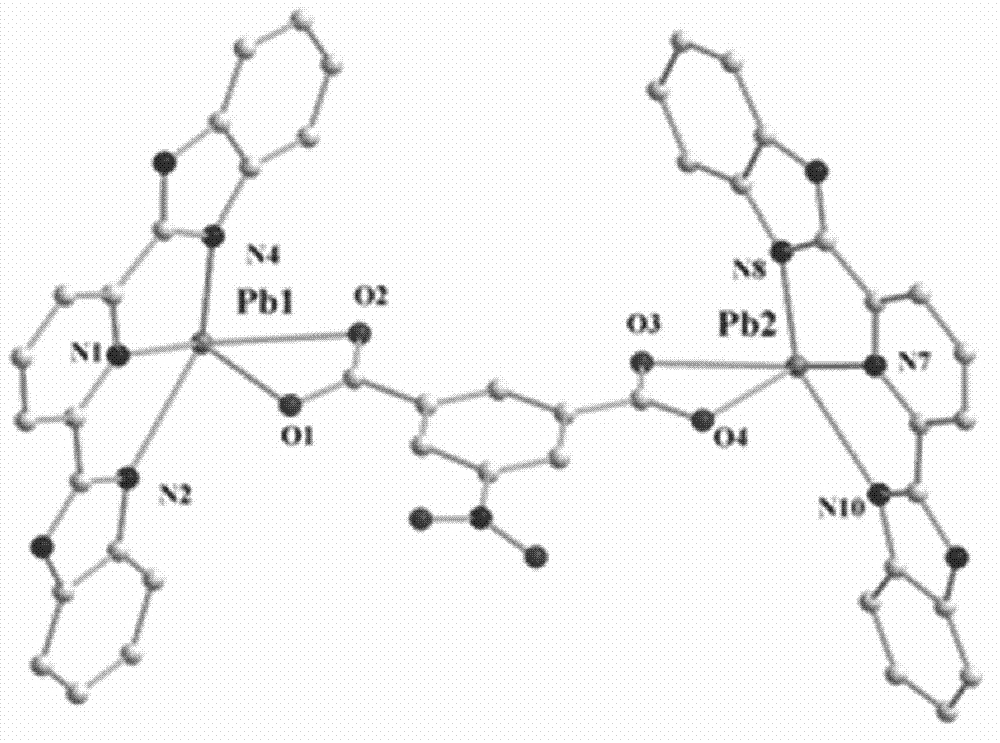
Benzimidazole pyridine complex and method for preparing same
A technology of benzimidazopyridines and benzimidazopyridines, applied in the field of anticancer compounds, can solve the problems of large toxic and side effects, long course of treatment, high toxicity and the like, and achieves simple and feasible method, strong antitumor activity and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] One, preparation method of the present invention:
[0051] (1) Preparation of 2,6-dibenzimidazolpyridine: Weigh 3.35 g of 2,6-dicarboxylic acid pyridine (20 mmol) and 0.476 g of o-phenylenediamine (44 mmol) as reactants, and use 50 mL of polymer Phosphoric acid is used as a solvent, and after being fully mixed, put it into a microwave rapid reaction system, and react at a power of 200w and a temperature of 100°C for 2 minutes; After the green reaction solution was cooled to room temperature, it was poured into 100 mL of ice water, and it turned into a pink turbid solution. It was neutralized with NaOH to pH=9~10, and a pink precipitate was produced. It was suction filtered once, and washed 3 times with distilled water to obtain the crude product. The crude product was recrystallized with methanol, filtered once with suction, washed with distilled water for 3 times, placed in a drying oven at 80°C, and baked for 20-30 minutes to obtain a white columnar compound which is...
Embodiment 2
[0072] One, preparation method of the present invention:
[0073] (1) Preparation of 2,6-dibenzimidazolpyridine: Weigh 3.35 g of 2,6-dicarboxylic acid pyridine (20 mmol) and 0.476 g of o-phenylenediamine (60 mmol) as reactants, and use 70 mL of polymer Phosphoric acid is used as a solvent. After fully mixing evenly, put it into a microwave rapid reaction system, and react at a power of 250w and a temperature of 110°C for 3 minutes; then react at a power of 350w and a temperature of 100°C for 5 minutes. After the green reaction solution was cooled to room temperature, it was poured into 100mL of ice water, and it turned into a pink turbid solution. It was neutralized with NaOH to pH=9~10, and a pink precipitate was produced. It was suction filtered once, washed twice with distilled water, and the obtained crude product. Recrystallize the crude product with methanol, filter it once with suction, wash it twice with distilled water, put it in a drying oven at 90°C, and bake it fo...
Embodiment 3
[0095] One, preparation method of the present invention:
[0096] (1) Preparation of 2,6-dibenzimidazolpyridine: Weigh 3.35 g of 2,6-dicarboxylic acid pyridine (20 mmol) and 0.476 g of o-phenylenediamine (40 mmol) as reactants, and use 50 mL of polymer Phosphoric acid is used as a solvent, and after being fully mixed, put it into a microwave rapid reaction system and react for 2 minutes at a power of 220w and a temperature of 80°C; then react for 7 minutes at a power of 320w and a temperature of 120°C. After the green reaction solution was cooled to room temperature, it was poured into 100mL of ice water, and it turned into a pink turbid solution. It was neutralized with NaOH to pH=9~10, and a pink precipitate was produced. It was suction filtered once, washed twice with distilled water, and the obtained crude product. Recrystallize the crude product with methanol, filter it with suction once, wash it twice with distilled water, put it in a drying oven at 80°C, and bake it fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com