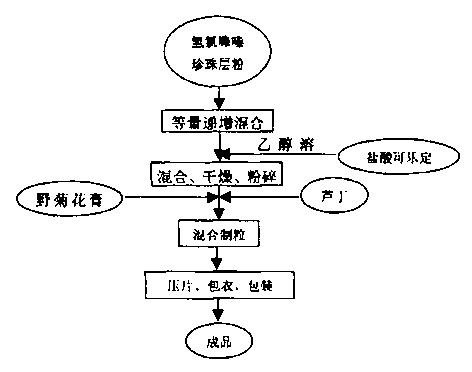
Preparation method of pearl layer powder-wild chrysanthemum cream powder hypotensive tablet
The technology of Zhenju Jiangya tablet and nacre powder is applied in the field of preparation of Zhenju Jiangya tablet, which can solve the problems of uneven mixing of clonidine hydrochloride, substandard content uniformity, etc., so as to improve bioavailability and increase batch size. Productivity, the effect of improving mixing uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
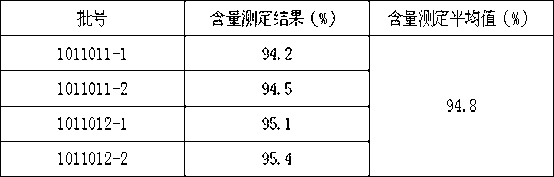
[0034] Example 1, a Zhenju Jiangya tablet (batch number: 101101, 10,000 tablets in total), the formula is:
[0035] Wild Chrysanthemum Cream Powder 1000g
[0036] Pearl layer powder 1000g
[0037] Clonidine Hydrochloride 300mg
[0038] Hydrochlorothiazide 50g
[0039] Rutin 200g
[0040] Its preparation method is as follows:
[0041] (1) Take 50.6g of hydrochlorothiazide and 999.7g of nacre powder and mix them in equal increments to obtain a mixed powder;
[0042] (2) Take another 0.295 g of clonidine hydrochloride, add ethanol with a volume concentration of 70% to dissolve, mix the obtained solution evenly into the above mixed powder, and stir repeatedly to obtain a mixture;
[0043] (3) Dry the above mixture at a temperature of 40-60°C, and pulverize it into powder in a pulverizer, and pass through a No. 5 sieve;
[0044] (4) Take 999.8g of wild chrysanthemum paste powder and 200.1g of rutin and add it to the above-mentioned powder passed through a No. 5 sieve, mix and...
Embodiment 2
[0065] Example 2, a Zhenju Jiangya tablet (batch number: 101102, 10,000 tablets in total), the formula is:
[0066] Wild Chrysanthemum Cream Powder 1000g
[0067] Pearl layer powder 1000g
[0068] Clonidine Hydrochloride 300mg
[0069] Hydrochlorothiazide 50g
[0070] Rutin 200g
[0071] Its preparation method is as follows:
[0072] (1) Take 50.7g of hydrochlorothiazide and 1000.1g of nacre powder and mix them in equal increments;
[0073] (2) Take another 0.312g of clonidine hydrochloride, add ethanol with a volume concentration of 70% to dissolve, mix the obtained solution evenly into the above mixed powder, and repeatedly stir the mixture;
[0074] (3) Dry the above mixture at a temperature of 40-60°C, and pulverize it into powder in a pulverizer;
[0075] (4) Add 1000.1g of wild chrysanthemum paste powder and 200.1g of rutin to the crushed powder, mix and granulate;
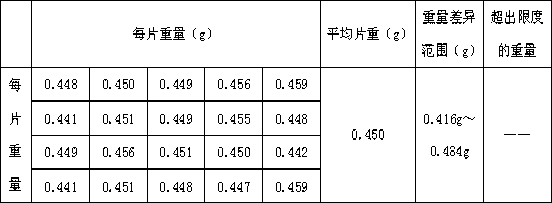
[0076] (5) Press into 10,000 tablets, and coat the compressed tablets, and the weight of the coati...
Embodiment 3
[0097] Example 3, a Zhenju Jiangya tablet (batch number: 101103, 10,000 tablets in total), the formula is:
[0098] Wild Chrysanthemum Cream Powder 1000g
[0099] Pearl layer powder 1000g
[0100] Clonidine Hydrochloride 300mg
[0101] Hydrochlorothiazide 50g
[0102] Rutin 200g
[0103] The preparation method is as follows:
[0104] (1) Take 49.9g of hydrochlorothiazide and 1000.4g of nacre powder and mix them in equal increments;
[0105] (2) Take another 0.312g of clonidine hydrochloride, add ethanol with a volume concentration of 70% to dissolve, mix the obtained solution evenly into the above mixed powder, and repeatedly stir the mixture;
[0106] (3) Dry the above mixture at a temperature of 40-60°C, and pulverize it into powder in a pulverizer;
[0107] (4) Add 1000.1g of wild chrysanthemum paste powder and 299.9g of rutin to the crushed powder, mix and granulate;
[0108] (5) Pressing into tablets and coating, the coating weight is 2% to 3% of the total weight o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com